Analysis of Total Knee Arthroplasty revision causes
- PMID: 29444666
- PMCID: PMC5813428
- DOI: 10.1186/s12891-018-1977-y
Analysis of Total Knee Arthroplasty revision causes
Abstract
Background: The number of revision Total Knee Arthroplasty (TKA) is rising in many countries. The aim of this study was the prospective assessment of the underlying causes leading to revision TKA in a tertiary care hospital and the comparison of those reasons with previously published data.
Methods: In this study patients who had revision TKA between 2010 and 2015 were prospectively included. Revision causes were categorized using all available information from patients' records including preoperative diagnostics, intraoperative findings as well as the results of the periprosthetic tissue analysis. According to previous studies patients were divided into early (up to 2 years) and late revision (more than 2 years). Additional also re-revisions after already performed revision TKA were included.
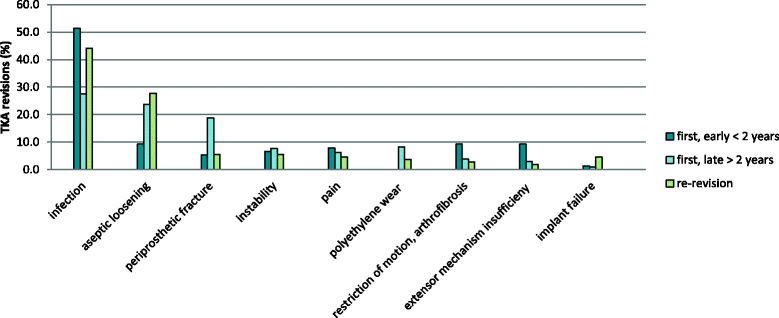
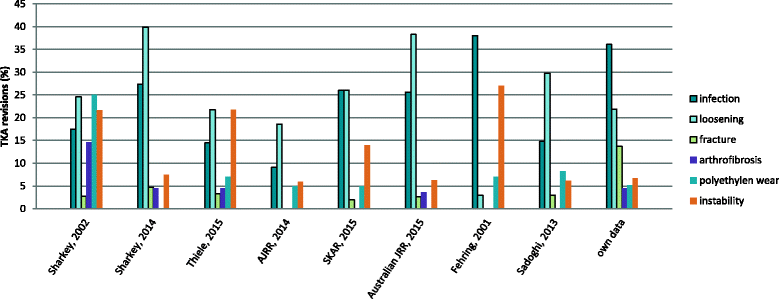
Results: We assessed 312 patients who underwent 402 revision TKA, 89.6% of them were referred to our center for revision surgery. In 289 patients (71.9%) this was the first revision surgery after primary TKA. Among the first revisions the majority was late revisions (73.7%). One hundred thirteen patients (28.1%) had already had one or more revision surgeries before. Overall, the most frequent reason for revision was infection (36.1%) followed by aseptic loosening (21.9%) and periprosthetic fracture (13.7%).
Conclusions: In a specialized arthroplasty center periprosthetic joint infection (PJI) was the most common reason for revision and re-revision TKA. This is in contrast to population-based registry data and has consequences on costs as well as on success rates in such centers.
Keywords: Complication; Failure; Re-revision; Revision; Total knee arthroplasty.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the local independent ethics committee and each patient signed an informed consent. An ethics approval for this study was obtained from the Ethics Committee of the University Medicine Carl Gustav Carus, TU Dresden in 2011 (EK 288082011).
Consent for publication
Not applicable.
Competing interests
Research support and other financial or material support have been received from the following companies: Arthrosehilfe, Aesculap, Mathys, Smith&Nephew, Stryker and Zimmer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Registry AJR. American Joint Replacement Registry. ISSN 2375-9119 (online). Annual Report 2014. 2014. www.ajrr.net.
-
- Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. Adelaide: AOA; 2015.
-
- Registry NJ. National Joint Registry. ISSN 2054-183X (Online). 12th Annual Report 2015 - National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2015. www.njrreports.org.uk.
-
- IQTIG. Institut für Qualitätssicherung und Transparenz im Gesundheitswesen, Berlin. ISBN 978-3-9818131-0-4. Qualitätsreport 2015. 2015. www.iqtig.org.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources