Whole bone testing in small animals: systematic characterization of the mechanical properties of different rodent bones available for rat fracture models
- PMID: 29444703
- PMCID: PMC5813325
- DOI: 10.1186/s40001-018-0307-z
Whole bone testing in small animals: systematic characterization of the mechanical properties of different rodent bones available for rat fracture models
Abstract
Objectives: Rat fracture models are extensively used to characterize normal and pathological bone healing. Despite, systematic research on inter- and intra-individual differences of common rat bones examined is surprisingly not available. Thus, we studied the biomechanical behaviour and radiological characteristics of the humerus, the tibia and the femur of the male Wistar rat-all of which are potentially available in the experimental situation-to identify useful or detrimental biomechanical properties of each bone and to facilitate sample size calculations.
Methods: 40 paired femura, tibiae and humeri of male Wistar rats (10-38 weeks, weight between 240 and 720 g) were analysed by DXA, pQCT scan and three-point-bending. Bearing and loading bars of the biomechanical setup were adapted percentually to the bone's length. Subgroups of light (skeletal immature) rats under 400 g (N = 11, 22 specimens of each bone) and heavy (mature) rats over 400 g (N = 9, 18 specimens of each bone) were formed and evaluated separately.
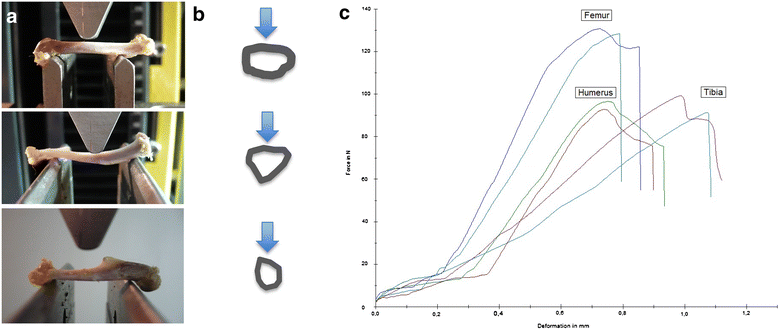
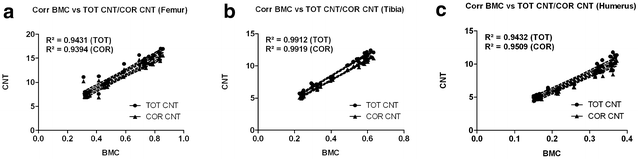
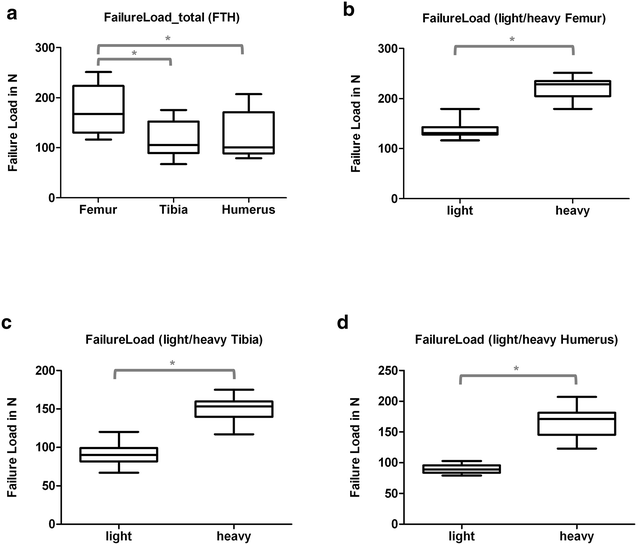
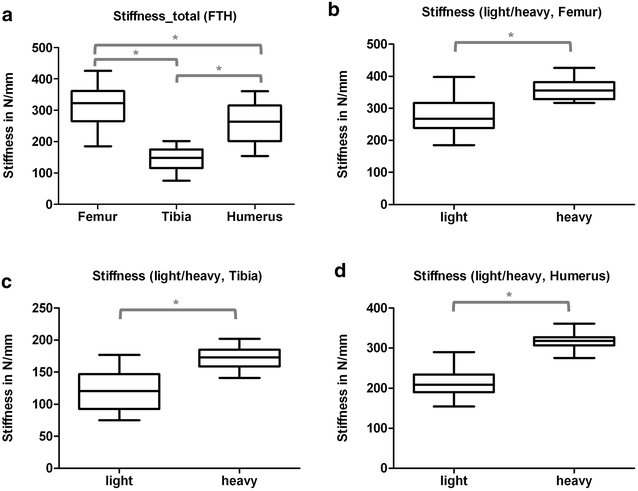
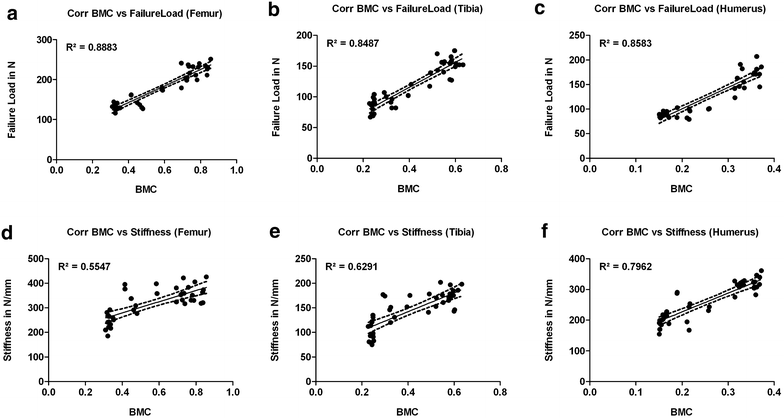
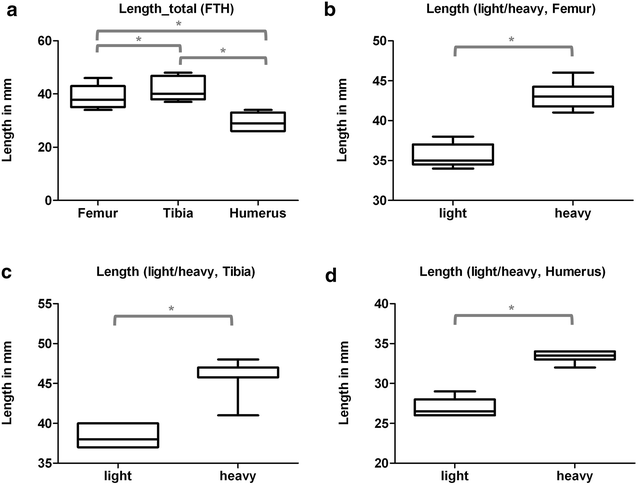
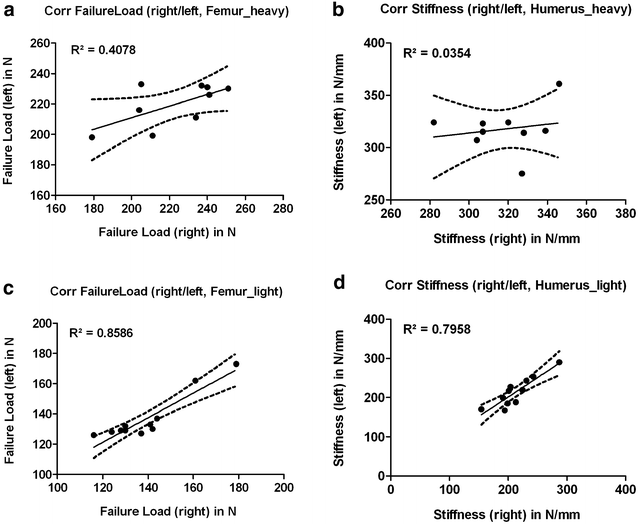
Results: Radiologically, neither significant differences between left and right bones, nor a specific side preference was evident. Mean side differences of the BMC were relatively small (1-3% measured by DXA and 2.5-5% by pQCT). Over all, bone mineral content (BMC) assessed by DXA and pQCT (TOT CNT, CORT CNT) showed high correlations between each other (BMC vs. TOT and CORT CNT: R2 = 0.94-0.99). The load-displacement diagram showed a typical, reproducible curve for each type of bone. Tibiae were the longest bones (mean 41.8 ± 4.12 mm) followed by femurs (mean 38.9 ± 4.12 mm) and humeri (mean 29.88 ± 3.33 mm). Failure loads and stiffness ranged from 175.4 ± 45.23 N / 315.6 ± 63.00 N/mm for the femurs, 124.6 ± 41.13 N / 260.5 ± 59.97 N/mm for the humeri to 117.1 ± 33.94 N / 143.8 ± 36.99 N/mm for the tibiae. Smallest interindividual differences were observed in failure loads of the femurs (CV% 8.6) and tibiae (CV% 10.7) of heavy animals, light animals showed good consistency in failure loads of the humeri (CV% 7.7). Most consistent results of both sides (left vs. right) in failure loads were provided by the femurs of light animals (mean difference 4.0 ± 2.8%); concerning stiffness, humeri of heavy animals were most consistent (mean difference of 6.2 ± 5%). In general, the failure loads showed strong correlations to the BMC (R2 = 0.85-0.88) whereas stiffness correlated only moderate, except for the humerus (BMC vs. stiffness: R2 = 0.79).
Discussion: Altogether, the rat's femur of mature specimens showed the most accurate and consistent radiological and biomechanical results. In synopsis with the common experimental use enabling comparison among different studies, this bone offers ideal biomechanical conditions for three point bending experiments. This can be explained by the combination of a superior aspect ratio and a round and long, straight morphology, which satisfies the beam criteria more than other bones tested.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

