Optimizing Hybrid Closed-Loop Therapy in Adolescents and Emerging Adults Using the MiniMed 670G System
- PMID: 29444895
- PMCID: PMC6463622
- DOI: 10.2337/dc17-1682
Optimizing Hybrid Closed-Loop Therapy in Adolescents and Emerging Adults Using the MiniMed 670G System
Abstract
Objective: The MiniMed 670G System is the first commercial hybrid closed-loop (HCL) system for management of type 1 diabetes. Using data from adolescent and young adult participants, we compared insulin delivery patterns and time-in-range metrics in HCL (Auto Mode) and open loop (OL). System alerts, usage profiles, and operational parameters were examined to provide suggestions for optimal clinical use of the system.
Research design and methods: Data from 31 adolescent and young adult participants (14-26 years old) at three clinical sites in the 670G pivotal trial were analyzed. Participants had a 2-week run-in period in OL, followed by a 3-month in-home study phase with HCL functionality enabled. Data were compared between baseline OL and HCL use after 1 week, 1 month, 2 months, and 3 months.
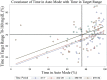
Results: Carbohydrate-to-insulin (C-to-I) ratios were more aggressive for all meals with HCL compared with baseline OL. Total daily insulin dose and basal-to-bolus ratio did not change during the trial. Time in range increased 14% with use of Auto Mode after 3 months (P < 0.001), and HbA1c decreased 0.75%. Auto Mode exits were primarily due to sensor/insulin delivery alerts and hyperglycemia. The percentage of time in Auto Mode gradually declined from 87%, with a final use rate of 72% (-15%).
Conclusions: In transitioning young patients to the 670G system, providers should anticipate immediate C-to-I ratio adjustments while also assessing active insulin time. Users should anticipate occasional Auto Mode exits, which can be reduced by following system instructions and reliably bolusing for meals. Unique 670G system functionality requires ongoing clinical guidance and education from providers.
© 2018 by the American Diabetes Association.
Figures
References
-
- Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther 2009;11(Suppl. 1):S113–S119 - PubMed
-
- Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015;38:1036–1043 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous