The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines
- PMID: 29444910
- PMCID: PMC5827037
- DOI: 10.1530/ERC-17-0445
The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines
Erratum in
-
The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines.Endocr Relat Cancer. 2018 Apr;25(4):X1-X2. doi: 10.1530/ERC-17-0445e. Endocr Relat Cancer. 2018. PMID: 29540494 Free PMC article. No abstract available.
Abstract
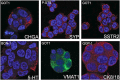
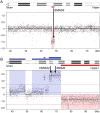
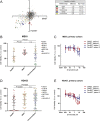
Experimental models of neuroendocrine tumour disease are scarce, and no comprehensive characterisation of existing gastroenteropancreatic neuroendocrine tumour (GEPNET) cell lines has been reported. In this study, we aimed to define the molecular characteristics and therapeutic sensitivity of these cell lines. We therefore performed immunophenotyping, copy number profiling, whole-exome sequencing and a large-scale inhibitor screening of seven GEPNET cell lines. Four cell lines, GOT1, P-STS, BON-1 and QGP-1, displayed a neuroendocrine phenotype while three others, KRJ-I, L-STS and H-STS, did not. Instead, these three cell lines were identified as lymphoblastoid. Characterisation of remaining authentic GEPNET cell lines by copy number profiling showed that GOT1, among other chromosomal alterations, harboured losses on chromosome 18 encompassing the SMAD4 gene, while P-STS had a loss on 11q. BON-1 had a homozygous loss of CDKN2A and CDKN2B, and QGP-1 harboured amplifications of MDM2 and HMGA2 Whole-exome sequencing revealed both disease-characteristic mutations (e.g. ATRX mutation in QGP-1) and, for patient tumours, rare genetic events (e.g. TP53 mutation in P-STS, BON-1 and QGP-1). A large-scale inhibitor screening showed that cell lines from pancreatic NETs to a greater extent, when compared to small intestinal NETs, were sensitive to inhibitors of MEK. Similarly, neuroendocrine NET cells originating from the small intestine were considerably more sensitive to a group of HDAC inhibitors. Taken together, our results provide a comprehensive characterisation of GEPNET cell lines, demonstrate their relevance as neuroendocrine tumour models and explore their therapeutic sensitivity to a broad range of inhibitors.
Keywords: GEPNET; cell lines; copy number alterations; exome sequencing; immunophenotyping; inhibitor screening; neuroendocrine tumour; trametinib; vorinostat.
© 2018 The authors.
Figures



References
-
- Abe N, Watanabe T, Suzuki Y, Matsumoto N, Masaki T, Mori T, Sugiyama M, Chiappetta G, Fusco A, Atomi Y. 2003. An increased high-mobility group A2 expression level is associated with malignant phenotype in pancreatic exocrine tissue. British Journal of Cancer 89 2104–2109. ( 10.1038/sj.bjc.6601391) - DOI - PMC - PubMed
-
- Andersson E, Arvidsson Y, Swärd C, Hofving T, Wängberg B, Kristiansson E, Nilsson O. 2016. Expression profiling of small intestinal neuroendocrine tumors identifies subgroups with clinical relevance, prognostic markers and therapeutic targets. Modern Pathology 29 616–629. ( 10.1038/modpathol.2016.48) - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

