Maraviroc Is Associated with Latent HIV-1 Reactivation through NF-κB Activation in Resting CD4+ T Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy
- PMID: 29444937
- PMCID: PMC5899181
- DOI: 10.1128/JVI.01931-17
Maraviroc Is Associated with Latent HIV-1 Reactivation through NF-κB Activation in Resting CD4+ T Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy
Abstract
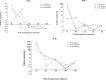
Maraviroc is a CCR5 antagonist used in the treatment of HIV-1 infection. We and others have suggested that maraviroc could reactivate latent HIV-1. To test the latency-reversing potential of maraviroc and the mechanisms involved, we performed a phase II, single-center, open-label study in which maraviroc was administered for 10 days to 20 HIV-1-infected individuals on suppressive antiretroviral therapy (EudraCT registration no. 2012-003215-66). All patients completed full maraviroc dosing and follow-up. The primary endpoint was to study whether maraviroc may reactivate HIV-1 latency, eliciting signaling pathways involved in the viral reactivation. An increase in HIV-1 transcription in resting CD4+ T cells, estimated by levels of HIV-1 unspliced RNA, was observed. Moreover, activation of the NF-κB transcription factor was observed in these cells. To elucidate the mechanism of NF-κB activation by maraviroc, we have evaluated in HeLa P4 C5 cells, which stably express CCR5, whether maraviroc could be acting as a partial CCR5 agonist, with no other mechanisms or pathways involved. Our results show that maraviroc can induce NF-κB activity and that NF-κB targets gene expression by CCR5 binding, since the use of TAK779, a CCR5 inhibitor, blocked NF-κB activation and functionality. Taking the results together, we show that maraviroc may have a role in the activation of latent virus transcription through the activation of NF-κB as a result of binding CCR5. Our results strongly support a novel use of maraviroc as a potential latency reversal agent in HIV-1-infected patients.IMPORTANCE HIV-1 persistence in a small pool of long-lived latently infected resting CD4+ T cells is a major barrier to viral eradication in HIV-1-infected patients on antiretroviral therapy. A potential strategy to cure HIV-1-infection is the use of latency-reversing agents to eliminate the reservoirs established in resting CD4+ T cells. As no drug has been shown to be completely effective so far, the search for new drugs and combinations remains a priority for HIV cure. We examined the ability of maraviroc, a CCR5 antagonist used as an antiretroviral drug, to activate latent HIV-1 in infected individuals on antiretroviral therapy. The study showed that maraviroc can activate NF-κB and, subsequently, induce latent HIV-1-transcription in resting CD4+ T cells from HIV-1-infected individuals on suppressive antiretroviral therapy. Additional interventions will be needed to eliminate latent HIV-1 infection. Our results suggest that maraviroc may be a new latency-reversing agent to interfere with HIV-1 persistence during antiretroviral therapy.
Keywords: HIV infected; HIV-1; LRA; NF-κB; latency; maraviroc; persistence; reservoir.
Copyright © 2018 American Society for Microbiology.
Figures




References
-
- Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, Hesselgesser J, Irrinki A, Murry JP, Stepan G, Stray KM, Tsai A, Yu H, Spindler J, Kearney M, Spina CA, McMahon D, Lalezari J, Sloan D, Mellors J, Geleziunas R, Cihlar T. 2014. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog 10:e1004071. doi:10.1371/journal.ppat.1004071. - DOI - PMC - PubMed
-
- Wightman F, Lu HK, Solomon AE, Saleh S, Harman AN, Cunningham AL, Gray L, Churchill M, Cameron PU, Dear AE, Lewin SR. 2013. Entinostat is a histone deacetylase inhibitor selective for class 1 histone deacetylases and activates HIV production from latently infected primary T cells. AIDS 27:2853–2862. doi:10.1097/QAD.0000000000000067. - DOI - PMC - PubMed
-
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi:10.1016/S2352-3018(14)70014-1. - DOI - PubMed
-
- Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sékaly R-P, Lewin SR. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi:10.1371/journal.ppat.1004473. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

