Rett syndrome: a neurological disorder with metabolic components
- PMID: 29445033
- PMCID: PMC5830535
- DOI: 10.1098/rsob.170216
Rett syndrome: a neurological disorder with metabolic components
Abstract
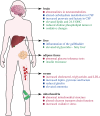
Rett syndrome (RTT) is a neurological disorder caused by mutations in the X-linked gene methyl-CpG-binding protein 2 (MECP2), a ubiquitously expressed transcriptional regulator. Despite remarkable scientific progress since its discovery, the mechanism by which MECP2 mutations cause RTT symptoms is largely unknown. Consequently, treatment options for patients are currently limited and centred on symptom relief. Thought to be an entirely neurological disorder, RTT research has focused on the role of MECP2 in the central nervous system. However, the variety of phenotypes identified in Mecp2 mutant mouse models and RTT patients implicate important roles for MeCP2 in peripheral systems. Here, we review the history of RTT, highlighting breakthroughs in the field that have led us to present day. We explore the current evidence supporting metabolic dysfunction as a component of RTT, presenting recent studies that have revealed perturbed lipid metabolism in the brain and peripheral tissues of mouse models and patients. Such findings may have an impact on the quality of life of RTT patients as both dietary and drug intervention can alter lipid metabolism. Ultimately, we conclude that a thorough knowledge of MeCP2's varied functional targets in the brain and body will be required to treat this complex syndrome.
Keywords: Rett syndrome; histone deacetylase; metabolism; methyl-CpG-binding protein 2; nuclear corepressor.
© 2018 The Authors.
Conflict of interest statement
M.J.J. is a scientific founder of ArRETT Neurosciences, Inc., a company designed to provide treatments to Rett syndrome patients.
Figures




References
-
- Rett A. 1966. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien. Med. Wochenschr. 1946, 723–726. - PubMed
-
- Hagberg B, Aicardi J, Dias K, Ramos O. 1983. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 14, 471–479. (doi:10.1002/ana.410140412) - DOI - PubMed
-
- Burd L, Randall T, Martsolf JT, Kerbeshian J. 1991. Rett syndrome symptomatology of institutionalized adults with mental retardation: comparison of males and females. Am. J. Ment. Retard. 95, 596–601. - PubMed
-
- Hagberg B. 2002. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 8, 61–65. (doi:10.1002/mrdd.10020) - DOI - PubMed
-
- Hagberg B, Witt-Engerström I, Opitz JM, Reynolds JF. 1986. Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am. J. Med. Genet. 25, 47–59. (doi:10.1002/ajmg.1320250506) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
