The carnitine system and cancer metabolic plasticity
- PMID: 29445084
- PMCID: PMC5833840
- DOI: 10.1038/s41419-018-0313-7
The carnitine system and cancer metabolic plasticity
Abstract
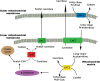
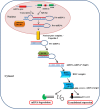
Metabolic flexibility describes the ability of cells to respond or adapt its metabolism to support and enable rapid proliferation, continuous growth, and survival in hostile conditions. This dynamic character of the cellular metabolic network appears enhanced in cancer cells, in order to increase the adaptive phenotype and to maintain both viability and uncontrolled proliferation. Cancer cells can reprogram their metabolism to satisfy the energy as well as the biosynthetic intermediate request and to preserve their integrity from the harsh and hypoxic environment. Although several studies now recognize these reprogrammed activities as hallmarks of cancer, it remains unclear which are the pathways involved in regulating metabolic plasticity. Recent findings have suggested that carnitine system (CS) could be considered as a gridlock to finely trigger the metabolic flexibility of cancer cells. Indeed, the components of this system are involved in the bi-directional transport of acyl moieties from cytosol to mitochondria and vice versa, thus playing a fundamental role in tuning the switch between the glucose and fatty acid metabolism. Therefore, the CS regulation, at both enzymatic and epigenetic levels, plays a pivotal role in tumors, suggesting new druggable pathways for prevention and treatment of human cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

