The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial
- PMID: 29445178
- PMCID: PMC5813237
- DOI: 10.1038/s41598-018-21241-z
The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial
Abstract
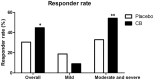
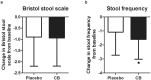
Irritable bowel syndrome (IBS) is a common disorder in gastrointestinal system and impairs the quality of life of the patients. Clostridium butyricum (CB) is a probiotics that has been used in several gastrointestinal diseases. The efficacy of CB in treating IBS is still unknown. This prospective, multi-centre, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy and safety of CB in treating diarrhea-predominant IBS (IBS-D) and analyze the fecal microbiota after treatment. Two hundred patients with IBS-D were recruited and were given CB or placebo for 4 weeks. End points included change from baseline in IBS symptoms, quality of life, stool consistency and frequency. Compared with placebo, CB is effective in improving the overall IBS-D symptoms (-62.12 ± 74.00 vs. -40.74 ± 63.67, P = 0.038) as well as quality of life (7.232 ± 14.06 vs. 3.159 ± 11.73, P = 0.032) and stool frequency (-1.602 ± 1.416 vs. -1.086 ± 1.644, P = 0.035). The responder rates are found higher in CB compared with the placebo (44.76% vs. 30.53%, P = 0.042). The change in fecal microbiota was analyzed and function pathways of CB in treating IBS-D were predicted. In conclusion, CB improves overall symptoms, quality of life and stool frequency in IBS-D patients and is considered to be used as a probiotics in treating IBS-D clinically.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial.J Clin Gastroenterol. 2012 Mar;46(3):220-7. doi: 10.1097/MCG.0b013e31823712b1. J Clin Gastroenterol. 2012. PMID: 22157240 Clinical Trial.
-
The efficacy of Bifidobacterium quadruple viable tablet in the treatment of diarrhea predominant irritable bowel syndrome: protocol for a randomized, double-blind, placebo-controlled, multicenter trial.Trials. 2020 Jun 30;21(1):597. doi: 10.1186/s13063-020-04490-0. Trials. 2020. PMID: 32605578 Free PMC article.
-
A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome.BMC Gastroenterol. 2018 May 25;18(1):71. doi: 10.1186/s12876-018-0788-9. BMC Gastroenterol. 2018. PMID: 29801486 Free PMC article. Clinical Trial.
-
Fecal microbiota transplantation in patients with irritable bowel syndrome: an overview of current studies.J Appl Microbiol. 2023 Mar 1;134(3):lxad044. doi: 10.1093/jambio/lxad044. J Appl Microbiol. 2023. PMID: 36882216 Review.
-
Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome.Aliment Pharmacol Ther. 2019 Aug;50(3):240-248. doi: 10.1111/apt.15330. Epub 2019 May 28. Aliment Pharmacol Ther. 2019. PMID: 31136009
Cited by
-
Electrospun Solid Formulation of Anaerobic Gut Microbiome Bacteria.AAPS PharmSciTech. 2020 Jul 31;21(6):214. doi: 10.1208/s12249-020-01769-y. AAPS PharmSciTech. 2020. PMID: 32737608 Free PMC article.
-
Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis.Nutrients. 2023 Sep 4;15(17):3856. doi: 10.3390/nu15173856. Nutrients. 2023. PMID: 37686889 Free PMC article.
-
A Pilot Study: Favorable Effects of Clostridium butyricum on Intestinal Microbiota for Adjuvant Therapy of Lung Cancer.Cancers (Basel). 2022 Jul 23;14(15):3599. doi: 10.3390/cancers14153599. Cancers (Basel). 2022. PMID: 35892858 Free PMC article.
-
Complete Genome Sequence of Clostridium butyricum Strain DKU_butyricum 4-1, Isolated from Infant Feces.Microbiol Resour Announc. 2020 Mar 5;9(10):e01341-19. doi: 10.1128/MRA.01341-19. Microbiol Resour Announc. 2020. PMID: 32139575 Free PMC article.
-
Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review.Biomolecules. 2021 Aug 4;11(8):1154. doi: 10.3390/biom11081154. Biomolecules. 2021. PMID: 34439821 Free PMC article. Review.
References
-
- Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Alimentary pharmacology & therapeutics. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. - DOI - PubMed
-
- Ki Cha B, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Journal of clinical gastroenterology. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. - DOI - PubMed
-
- American College of Gastroenterology Task Force on Irritable Bowel, S. et al. An evidence-based position statement on the management of irritable bowel syndrome. The American journal of gastroenterology. 2009;104(Suppl 1):S1–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

