In vitro cytotoxicity activity of novel Schiff base ligand-lanthanide complexes
- PMID: 29445233
- PMCID: PMC5812993
- DOI: 10.1038/s41598-018-21366-1
In vitro cytotoxicity activity of novel Schiff base ligand-lanthanide complexes
Abstract
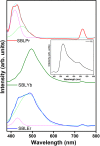
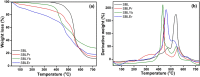
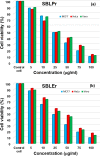
A Schiff base ligand (SBL), N2, N3-bis (anthracen-9-ylmethylene) pyridine-2, 3-diamine, was synthesized through the condensation of 2,6-diaminopyridine and anthracene-9-carbaldehyde using a 1:2 ratio. 1H NMR spectra confirmed the observation of non-involvement aromatic carboxylic proton in SBL. A novel series of lanthanide (i.e., praseodymium (Pr), erbium (Er), and ytterbium (Yb))-based SBL metal complexes was successfully synthesized, and their functional groups were elaborately demonstrated using UV-visible, Fourier transform infrared (FT-IR), and fluorescence spectroscopy analyses. FT-IR spectral studies revealed that SBL behaved as a bidentate ligand and it was structured with metal ions by the two azomethine nitrogens. The synthesized SBL-based metal complexes were elaborately performed for cytotoxicity activity versus Vero, human breast cancer (MCF7), and cervical (HeLa) anticancer cell lines.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Woollins JD, Woollins A, Rosenberg B. The detection of trace amounts of trans-Pt(NH3)2Cl2 in the presence of cis-Pt(NH3)2Cl2. A high performance liquid chromatographic application of kurnakow’s test. Polyhedron. 1983;2:175–178. doi: 10.1016/S0277-5387(00)83954-6. - DOI
-
- Kathiresan S, Mugesh S, Annaraj J, Murugan M. Mixed-ligand copper(II) Schiff base complexes: the vital role of co-ligands in DNA/protein interactions and cytotoxicity. New J. Chem. 2017;41:1267–1283. doi: 10.1039/C6NJ03501A. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

