An open-label, prospective interventional study of the tolerability and efficacy of 0.4 mg oral tamsulosin oral controlled absorption system in men with lower urinary tract symptoms associated with benign prostatic hyperplasia who are unsatisfied with treatment with 0.2 mg tamsulosin
- PMID: 29445269
- PMCID: PMC5808696
- DOI: 10.2147/CIA.S152701
An open-label, prospective interventional study of the tolerability and efficacy of 0.4 mg oral tamsulosin oral controlled absorption system in men with lower urinary tract symptoms associated with benign prostatic hyperplasia who are unsatisfied with treatment with 0.2 mg tamsulosin
Abstract
Purpose: The aim of this study was to investigate the efficacy and tolerability of switching from 0.2 mg tamsulosin to 0.4 mg tamsulosin oral controlled absorption system (OCAS) over a 12-week period in Taiwanese men with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH).
Patients and methods: Taiwanese male patients who were dissatisfied with treatment with 0.2 mg tamsulosin were enrolled in this clinical study and switched to 0.4 mg tamsulosin OCAS. Efficacy was assessed over a 12-week period by an International Prostate Symptom Score (IPSS) questionnaire and analysis of urinary flow by uroflowmetry.
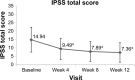
Results: A statistically significant improvement was observed in total IPSS scores from baseline (14.94±7.41, moderate) to 12 weeks (7.36±5.77, mild) in 81 patients who were switched from 0.2 to 0.4 mg tamsulosin OCAS (P<0.001). The IPSS subscores for storage, voiding, nocturia, and quality of life (QOL) were also significantly improved over the 12-week period. Uroflowmetry analysis demonstrated significantly increased maximum flow rate, average flow rate, and mean voided volume from baseline to the end of the 12-week period. The 0.4 mg tamsulosin OCAS dose was well tolerated, with only mild dizziness (five patients) and headache (two patients) as the most frequent adverse events. No clinically significant reduction was observed in blood pressure or vital signs.
Conclusion: Treatment with 0.4 mg tamsulosin OCAS in Taiwanese men with LUTS associated with BPH who were dissatisfied with 0.2 mg tamsulosin significantly improved IPSS scores, urinary flow, and QOL and was well tolerated, suggesting that this should be the recommended dose offered to Taiwanese male patients.
Keywords: LUTS; neoplasms; outcomes; prostate; α1-adrenergic receptor.
Conflict of interest statement
Disclosure C-LC, C-PH, Y-HL, and K-HT received investigator fees from Astellas Taiwan Pharma, Inc., for conducting the study. The authors report no other conflicts of interest in this work.
Figures


References
-
- Lee C, Kozlowski JM, Grayhack JT. Intrinsic and extrinsic factors controlling benign prostatic growth. Prostate. 1997;31(2):131–138. - PubMed
-
- Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338(8765):469–471. - PubMed
-
- Barkin J. Benign prostatic hyperplasia and lower urinary tract symptoms: evidence and approaches for best case management. Can J Urol. 2011;18(Suppl):14–19. - PubMed
-
- van Koeveringe GA, Vahabi B, Andersson KE, Kirschner-Herrmans R, Oelke M. Detrusor underactivity: a plea for new approaches to a common bladder dysfunction. Neurourol Urodyn. 2011;30(5):723–728. - PubMed
-
- Oelke M, Baard J, Wijkstra H, de la Rosette JJ, Jonas U, Hofner K. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol. 2008;54(2):419–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

