Effects of silica-gentamicin nanohybrids on osteogenic differentiation of human osteoblast-like SaOS-2 cells
- PMID: 29445277
- PMCID: PMC5810519
- DOI: 10.2147/IJN.S147849
Effects of silica-gentamicin nanohybrids on osteogenic differentiation of human osteoblast-like SaOS-2 cells
Abstract
Introduction: In recent years, there has been an increasing interest in silica (SiO2) nanoparticles (NPs) as drug delivery systems. This interest is mainly attributed to the ease of their surface functionalization for drug loading. In orthopedic applications, gentamicin-loaded SiO2 NPs (nanohybrids) are frequently utilized for their prolonged antibacterial effects. Therefore, the possible adverse effects of SiO2-gentamicin nanohybrids on osteogenesis of bone-related cells should be thoroughly investigated to ensure safe applications.
Materials and methods: The effects of SiO2-gentamicin nanohybrids on the cell viability and osteogenic differentiation of human osteoblast-like SaOS-2 cells were investigated, together with native SiO2 NPs and free gentamicin.
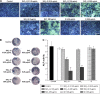
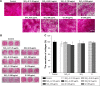
Results: The results of Cell Count Kit-8 (CCK-8) assay show that both SiO2-gentamicin nanohybrids and native SiO2 NPs reduce cell viability of SaOS-2 cells in a dose-dependent manner. Regarding osteogenesis, SiO2-gentamicin nanohybrids and native SiO2 NPs at the concentration range of 31.25-125 μg/mL do not influence the osteogenic differentiation capacity of SaOS-2 cells. At a high concentration (250 μg/mL), both materials induce a lower expression of alkaline phosphatase (ALP) but an enhanced mineralization. Free gentamicin at concentrations of 6.26 and 9.65 μg/mL does not significantly influence the cell viability and osteogenic differentiation capacity of SaOS-2 cells.
Conclusions: The results of this study suggest that both SiO2-gentamicin nanohybrids and SiO2 NPs show cytotoxic effects to SaOS-2 cells. Further investigation on the effects of SiO2-gentamicin nanohybrids on the behaviors of stem cells or other regular osteoblasts should be conducted to make a full evaluation of the safety of SiO2-gentamicin nanohybrids in orthopedic applications.
Keywords: ALP activity; SiO2 NPs; cytotoxicity; gentamicin; mineralization.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Choi E, Kim S. How can doxorubicin loading orchestrate in vitro degradation behaviors of mesoporous silica nanoparticles under a physiological condition? Langmuir. 2017;33(20):4974–4980. - PubMed
-
- Park MV, Verharen HW, Zwart E, et al. Genotoxicity evaluation of amorphous silica nanoparticles of different sizes using the micronucleus and the plasmid lacZ gene mutation assay. Nanotoxicology. 2011;5(2):168–181. - PubMed
-
- Qian J, Wang D, Cai F, Zhan Q, Wang Y, He S. Photosensitizer encapsulated organically modified silica nanoparticles for direct two-photon photodynamic therapy and in vivo functional imaging. Biomaterials. 2012;33(19):4851–4860. - PubMed
-
- Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41(7):2590–2605. - PubMed
-
- Lee JE, Lee N, Kim T, Kim J, Hyeon T. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res. 2011;44(10):893–902. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

