Clinical utility of ustekinumab in Crohn's disease
- PMID: 29445293
- PMCID: PMC5810512
- DOI: 10.2147/JIR.S157358
Clinical utility of ustekinumab in Crohn's disease
Abstract
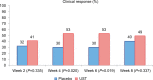
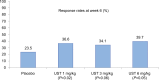
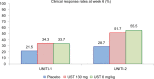
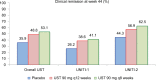
The introduction of anti-tumor necrosis factor (TNF) therapy marked an important milestone in the management of moderate-to-severe Crohn's disease (CD). However, there remains a pressing demand for alternative therapeutic options for patients with primary nonresponse, secondary loss of response, or intolerable side effects to conventional treatment and TNF antagonists. Ustekinumab (UST) is a fully human IgG1κ monoclonal antibody that inhibits the p40 subunit shared by the proinflammatory cytokines, the interleukin (IL)-12 and -23. This blockade leads to dampening of the inflammatory cascade and differentiation of inflammatory T cells. The clinical development program for UST in CD includes dose finding Phase II (Crohn's Evaluation of Response to Ustekinumab Anti-Interleukin-12/23 for Induction [CERTIFI]) and the pivotal Phase III (UNITI) trials that demonstrated both the clinical efficacy and safety in anti-TNF-naive and anti-TNF-exposed patients. Real-world evidence has further defined the role of UST in CD management. In this review, we discuss the mechanism of action of UST, describe the results of the randomized controlled trials with this agent, and review the real-world efficacy and safety data from observational cohorts. Finally, we identify areas of future research in the IL-12/23 inflammatory pathway and discuss the positioning of this novel therapeutic option in CD treatment algorithms.
Keywords: Crohn’s disease; interleukin; ustekinumab.
Conflict of interest statement
Disclosure CM is supported in part by the Canadian Institutes of Health Research and the Canadian Association of Gastroenterology. PGK has received consulting fees from AbbVie, Takeda, and Pfizer and speaker’s bureau fees from AbbVie, Takeda, Ferring, Pfizer, UCB, and Janssen. RP has received scientific advisory board fees from Abbott/AbbVie, Amgen, Janssen, Merck, Pfizer, Prometheus Laboratories, Salix Pharma, Shire, Takeda, and Warner Chilcott, consulting fees from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Elan/Biogen, Eisai, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus Therapeutics and Diagnostics, Schering-Plough, Shire, Takeda, UCB Pharma, and Warner Chilcott, research grants from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Eisai, Elan/Biogen, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus, Shire, Schering-Plough, Takeda, UCB Pharma, and Warner Chilcott, and speaker’s bureau fees from Abbott/AbbVie, Amgen, Aptalis, Astra Zeneca, Baxter, BMS, Centocor, Eisai, Elan/Biogen, Ferring, GSK, Janssen, Merck, Millennium, Pfizer, Proctor & Gamble, Prometheus, Schering-Plough, Shire, Takeda, UCB Pharma, and Warner Chilcott. The authors report no other conflicts of interest in this work.
Figures

References
-
- Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–1605. - PubMed
-
- Gomollon F, Dignass A, Annese V, et al. ECCO 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25. - PubMed
-
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. - PubMed
-
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

