Ex Vivo Expanded Human Non-Cytotoxic CD8+CD45RClow/- Tregs Efficiently Delay Skin Graft Rejection and GVHD in Humanized Mice
- PMID: 29445370
- PMCID: PMC5797797
- DOI: 10.3389/fimmu.2017.02014
Ex Vivo Expanded Human Non-Cytotoxic CD8+CD45RClow/- Tregs Efficiently Delay Skin Graft Rejection and GVHD in Humanized Mice
Abstract
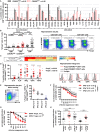
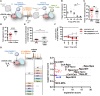
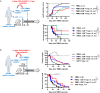
Both CD4+ and CD8+ Tregs play a critical role in the control of immune responses and immune tolerance; however, our understanding of CD8+ Tregs is limited while they are particularly promising for therapeutic application. We report here existence of highly suppressive human CD8+CD45RClow/- Tregs expressing Foxp3 and producing IFNγ, IL-10, IL-34, and TGFβ to mediate their suppressive activity. We demonstrate that total CD8+CD45RClow/- Tregs can be efficiently expanded in the presence of anti-CD3/28 mAbs, high-dose IL-2 and IL-15 and that such expanded Tregs efficiently delay GVHD and human skin transplantation rejection in immune humanized mice. Robustly expanded CD8+ Tregs displayed a specific gene signature, upregulated cytokines and expansion in the presence of rapamycin greatly improved proliferation and suppression. We show that CD8+CD45RClow/- Tregs are equivalent to canonical CD4+CD25highCD127low/- Tregs for suppression of allogeneic immune responses in vitro. Altogether, our results open new perspectives to tolerogenic strategies in human solid organ transplantation and GVHD.
Keywords: GVHD; NSG mice; Treg; cell therapy; graft; tolerance; transplantation.
Figures



References
-
- Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation (2015) 131:560–70. 10.1161/CIRCULATIONAHA.114.010988 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

