The Immunome in Two Inherited Forms of Pulmonary Fibrosis
- PMID: 29445374
- PMCID: PMC5797737
- DOI: 10.3389/fimmu.2018.00076
The Immunome in Two Inherited Forms of Pulmonary Fibrosis
Abstract
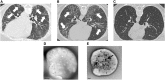
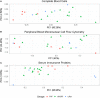
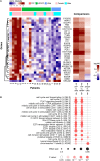
The immunome (immune cell phenotype, gene expression, and serum cytokines profiling) in pulmonary fibrosis is incompletely defined. Studies focusing on inherited forms of pulmonary fibrosis provide insights into mechanisms of fibrotic lung disease in general. To define the cellular and molecular immunologic phenotype in peripheral blood, high-dimensional flow cytometry and large-scale gene expression of peripheral blood mononuclear cells and serum proteomic multiplex analyses were performed and compared in a cohort with familial pulmonary fibrosis (FPF), an autosomal dominant disorder with incomplete penetrance; Hermansky-Pudlak syndrome pulmonary fibrosis (HPSPF), a rare autosomal recessive disorder; and their unaffected relatives. Our results showed high peripheral blood concentrations of activated central memory helper cells in patients with FPF. Proportions of CD38+ memory CD27- B-cells, IgA+ memory CD27+ B-cells, IgM+ and IgD+ B-cells, and CD39+ T helper cells were increased whereas those of CD39- T helper cells were reduced in patients affected with either familial or HPSPF. Gene expression and serum proteomic analyses revealed enrichment of upregulated genes associated with mitosis and cell cycle control in circulating mononuclear cells as well as altered levels of several analytes, including leptin, cytokines, and growth factors. In conclusion, dysregulation of the extra-pulmonary immunome is a phenotypic feature of FPF or HPSPF. Further studies investigating the blood immunome are indicated to determine the role of immune system dysregulation in the pathogenesis of pulmonary fibrosis.
Clinical trial registration: www.ClinicalTrials.gov, identifiers NCT00968084, NCT01200823, NCT00001456, and NCT00084305.
Keywords: B-cell; Hermansky–Pudlak syndrome; T-cell; cytokine; immunome; lymphocyte; pulmonary fibrosis; telomere disease.
Figures


References
-
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med (2015) 192:e3–19.10.1164/rccm.201506-1063ST - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

