The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy
- PMID: 29445380
- PMCID: PMC5797779
- DOI: 10.3389/fimmu.2018.00151
The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy
Abstract
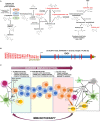
Tumors are composed of abnormally transformed cell types and tissues that differ from normal tissues in their genetic and epigenetic makeup, metabolism, and immunology. Molecular compounds that modulate the immune response against neoplasms offer promising new strategies to combat cancer. Inhibitors targeting the indoleamine-2,3-dioxygenase 1 enzyme (IDO1) represent one of the most potent therapeutic opportunities to inhibit tumor growth. Herein, we assess the biochemical role of IDO1 in tumor metabolism and immune surveillance, and review current diagnostic and therapeutic approaches that are intended to increase the effectiveness of immunotherapies against highly aggressive and difficult-to-treat IDO-expressing cancers.
Keywords: cancer diagnostics; clinical trial; gene expression; immune surveillance; immunotherapy; indoleamine-2,3-dioxygenase; metabolism.
Figures
References
-
- Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci (2017) 3:52–6.10.1016/j.jons.2017.04.001 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

