Approaches and Recent Developments for the Commercial Production of Semi-synthetic Artemisinin
- PMID: 29445390
- PMCID: PMC5797932
- DOI: 10.3389/fpls.2018.00087
Approaches and Recent Developments for the Commercial Production of Semi-synthetic Artemisinin
Abstract
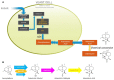
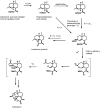
The antimalarial drug artemisinin is a natural product produced by the plant Artemisia annua. Extracts of A. annua have been used in Chinese herbal medicine for over two millennia. Following the re-discovery of A. annua extract as an effective antimalarial, and the isolation and structural elucidation of artemisinin as the active agent, it was recommended as the first-line treatment for uncomplicated malaria in combination with another effective antimalarial drug (Artemisinin Combination Therapy) by the World Health Organization (WHO) in 2002. Following the WHO recommendation, the availability and price of artemisinin fluctuated greatly, ranging from supply shortfalls in some years to oversupply in others. To alleviate these supply and price issues, a second source of artemisinin was sought, resulting in an effort to produce artemisinic acid, a late-stage chemical precursor of artemisinin, by yeast fermentation, followed by chemical conversion to artemisinin (i.e., semi-synthesis). Engineering to enable production of artemisinic acid in yeast relied on the discovery of A. annua genes encoding artemisinic acid biosynthetic enzymes, and synthetic biology to engineer yeast metabolism. The progress of this effort, which resulted in semi-synthetic artemisinin entering commercial production in 2013, is reviewed with an emphasis on recent publications and opportunities for further development. Aspects of both the biology of artemisinin production in A. annua, and yeast strain engineering are discussed, as are recent developments in the chemical conversion of artemisinic acid to artemisinin.
Keywords: Artemisia annua; Saccharomyces cerevisiae; artemisinic acid; artemisinin; semi-synthetic; synthetic biology.
Figures
References
-
- Acton N., Roth R. J. (1992). On the conversion of dihydroartemisinic acid into artemisinin. J. Org. Chem. 57 3610–3614. 10.1021/jo00039a020 - DOI
-
- Benjamin K. R., Silva I. R., Cherubim J. P., McPhee D., Paddon C. J. (2016). Developing commercial production of semi-synthetic artemisinin, and of β-Farnesene, an isoprenoid produced by fermentation of Brazilian sugar. J. Braz. Chem. Soc. 27 1339–1345. 10.5935/0103-5053.20160119 - DOI
-
- Boehme K., Brauer H.-D. (1992). Generation of singlet oxygen from hydrogen peroxide disproportionation catalyzed by molybdate ions. Inorg. Chem. 31 3468–3471. 10.1021/ic00042a024 - DOI
-
- Burgard A., Gieshoff T., Peschl A., Hörstermann D., Keleschovsky C., Villa R., et al. (2016). Optimisation of the photochemical oxidation step in the industrial synthesis of artemisinin. Chem. Eng. J. 294 83–96. 10.1016/j.cej.2016.02.085 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

