In Vivo Observation of Structural Changes in Neocortical Catecholaminergic Projections in Response to Drugs of Abuse
- PMID: 29445765
- PMCID: PMC5810039
- DOI: 10.1523/ENEURO.0071-17.2018
In Vivo Observation of Structural Changes in Neocortical Catecholaminergic Projections in Response to Drugs of Abuse
Abstract
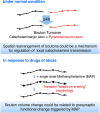
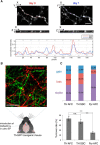
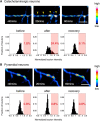
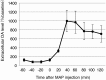
Catecholaminergic (dopamine and norepinephrine) projections to the cortex play an important role in cognitive functions and dysfunctions including learning, addiction, and mental disorders. While dynamics of glutamatergic synapses have been well studied in such contexts, little is known regarding catecholaminergic projections, owing to lack of robust methods. Here we report a system to monitor catecholaminergic projections in vivo over the timeframes that such events occur. Green fluorescent protein (GFP) expression driven by tyrosine hydroxylase promoter in a transgenic mouse line enabled us to perform two-photon imaging of cortical catecholaminergic projections through a cranial window. Repetitive imaging of the same axons over 24 h revealed the highly dynamic nature of catecholaminergic boutons. Surprisingly, administration of single high dose methamphetamine (MAP) induced a transient increase in bouton volumes. This new method opens avenues for longitudinal in vivo evaluation of structural changes at single release sites of catecholamines in association with physiology and pathology of cortical functions.
Keywords: axon; catecholamine; in vivo imaging; methamphetamine; neocortex; transgenic mouse.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
