Sodium lactate improves renal microvascular thrombosis compared to sodium bicarbonate and 0.9% NaCl in a porcine model of endotoxic shock: an experimental randomized open label controlled study
- PMID: 29445877
- PMCID: PMC5812960
- DOI: 10.1186/s13613-018-0367-9
Sodium lactate improves renal microvascular thrombosis compared to sodium bicarbonate and 0.9% NaCl in a porcine model of endotoxic shock: an experimental randomized open label controlled study
Abstract
Background: Sodium lactate seemed to improve fluid balance and avoid fluid overload. The objective of this study was to determine if these beneficial effects can be at least partly explained by an improvement in disseminated intravascular coagulation (DIC)-associated renal microvascular thrombosis.
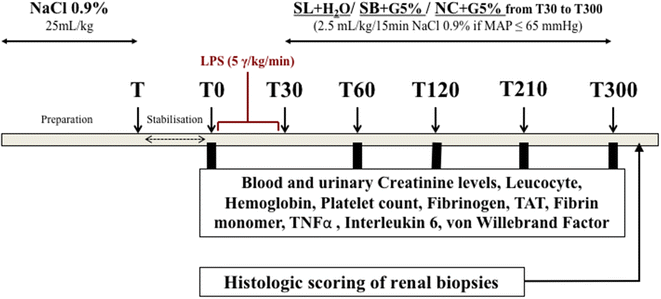
Methods: Ancillary work of an interventional randomized open label controlled experimental study. Fifteen female "Large White" pigs (2 months old) were challenged with intravenous infusion of E. coli endotoxin. Three groups of five animals were randomly assigned to receive different fluids: a treatment group received sodium lactate 11.2% (SL group); an isotonic control group received 0.9% NaCl (NC group); a hypertonic control group, with the same amount of osmoles and sodium than SL group, received sodium bicarbonate 8.4% (SB group). Glomerular filtration rate (GFR) markers, coagulation and inflammation parameters were measured over a 5-h period. Immediately after euthanasia, kidneys were withdrawn for histological study. Statistical analysis was performed with nonparametric tests and the Dunn correction for multiple comparisons. A p < 0.05 was considered significant.
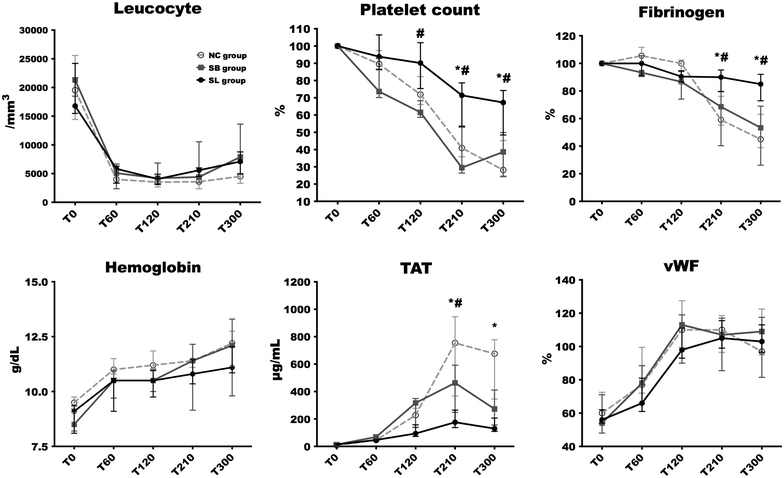
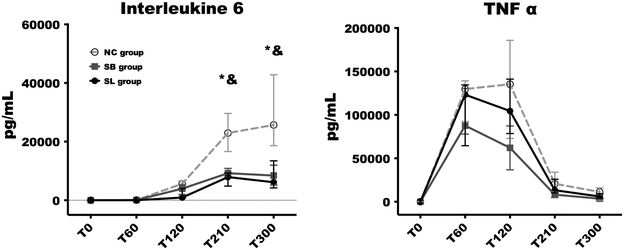
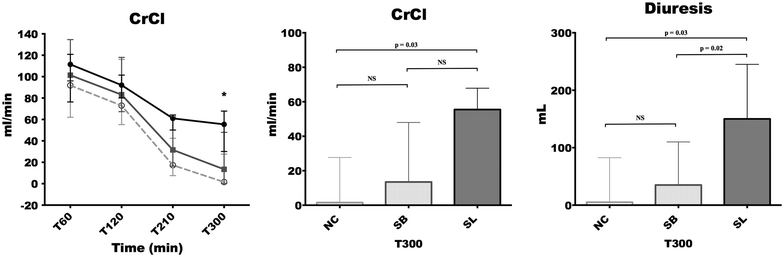
Results: The direct immunofluorescence study revealed that the percentage of capillary sections thrombosed in glomerulus were significantly lesser in SL group [5 (0-28) %] compared to NC [64 (43-79) %, p = 0.01] and SB [64 (43-79), p = 0.03] groups. Alterations in platelet count and fibrinogen level occurred earlier and were significantly more pronounced in both control groups compared to SL group (p < 0.05 at 210 and 300 min). The increase in thrombin-antithrombin complexes was significantly higher in NC [754 (367-945) μg/mL; p = 0.03] and SB [463 (249-592) μg/mL; p = 0.03] groups than in SL group [176 (37-265) μg/mL]. At the end of the experiment, creatinine clearance was significantly higher in SL group [55.46 (30.07-67.85) mL/min] compared to NC group [1.52 (0.17-27.67) mL/min, p = 0.03].
Conclusions: In this study, we report that sodium lactate improves DIC-associated renal microvascular thrombosis and preserves GFR. These findings could at least partly explain the better fluid balance observed with sodium lactate infusion.
Keywords: Disseminated intravascular coagulation; Fluid resuscitation; Glomerular filtration rate; Lactate infusion; Renal histology; Septic shock.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

