Conditional deletion of Cadherin 13 perturbs Golgi cells and disrupts social and cognitive behaviors
- PMID: 29446202
- PMCID: PMC6635760
- DOI: 10.1111/gbb.12466
Conditional deletion of Cadherin 13 perturbs Golgi cells and disrupts social and cognitive behaviors
Abstract
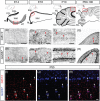
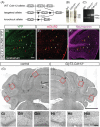
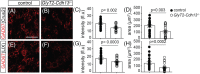
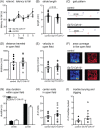
Inhibitory interneurons mediate the gating of synaptic transmission and modulate the activities of neural circuits. Disruption of the function of inhibitory networks in the forebrain is linked to impairment of social and cognitive behaviors, but the involvement of inhibitory interneurons in the cerebellum has not been assessed. We found that Cadherin 13 (Cdh13), a gene implicated in autism spectrum disorder and attention-deficit hyperactivity disorder, is specifically expressed in Golgi cells within the cerebellar cortex. To assess the function of Cdh13 and utilize the manipulation of Cdh13 expression in Golgi cells as an entry point to examine cerebellar-mediated function, we generated mice carrying Cdh13-floxed alleles and conditionally deleted Cdh13 with GlyT2::Cre mice. Loss of Cdh13 results in a decrease in the expression/localization of GAD67 and reduces spontaneous inhibitory postsynaptic current (IPSC) in cerebellar Golgi cells without disrupting spontaneous excitatory postsynaptic current (EPSC). At the behavioral level, loss of Cdh13 in the cerebellum, piriform cortex and endopiriform claustrum have no impact on gross motor coordination or general locomotor behaviors, but leads to deficits in cognitive and social abilities. Mice lacking Cdh13 exhibit reduced cognitive flexibility and loss of preference for contact region concomitant with increased reciprocal social interactions. Together, our findings show that Cdh13 is critical for inhibitory function of Golgi cells, and that GlyT2::Cre-mediated deletion of Cdh13 in non-executive centers of the brain, such as the cerebellum, may contribute to cognitive and social behavioral deficits linked to neurological disorders.
Keywords: Cadherin 13; GABAergic interneurons; GlyT2; Golgi cells; autism spectrum disorder; cerebellum; cognitive flexibility; reciprocal social interaction.
© 2018 The Authors. Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no potential conflict of interests.
Figures


References
-
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272‐303. - PubMed
-
- Berquin PC, Giedd JN, Jacobsen LK, et al. Cerebellum in attention‐deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087‐1093. - PubMed
-
- Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121‐130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

