Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin
- PMID: 29446523
- PMCID: PMC5969059
- DOI: 10.1111/dom.13258
Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: Post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin
Abstract
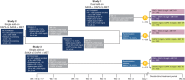
The safety of triple oral therapy with dapagliflozin plus saxagliptin plus metformin versus dual therapy with dapagliflozin or saxagliptin plus metformin was compared in a post-hoc analysis of 3 randomized trials of sequential or concomitant add-on of dapagliflozin and saxagliptin to metformin. In the concomitant add-on trial, patients with type 2 diabetes on stable metformin received dapagliflozin 10 mg/d plus saxagliptin 5 mg/d. In sequential add-on trials, patients on metformin plus either saxagliptin 5 mg/d or dapagliflozin 10 mg/d received dapagliflozin 10 mg/d or saxagliptin 5 mg/d, respectively, as add-on therapy. After 24 weeks, incidences of adverse events and serious adverse events were similar between triple and dual therapy and between concomitant and sequential add-on regimens. Urinary tract infections were more common with sequential than with concomitant add-on therapy; genital infections were reported only with sequential add-on of dapagliflozin to saxagliptin plus metformin. Hypoglycaemia incidence was <2.0% across all analysis groups. In conclusion, the safety and tolerability of triple therapy with dapagliflozin, saxagliptin and metformin, as either concomitant or sequential add-on, were similar to dual therapy with either agent added to metformin.
Keywords: DPP-4 inhibitor; SGLT-2 inhibitor; dapagliflozin; metformin; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
R. G. S., N. I., E. J. and H. C. are employees of AstraZeneca and L. H. is an employee of Bristol‐Myers Squibb. S. D. P. serves or has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Hanmi Pharmaceuticals, Intarcia, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi, Servier and Takeda and serves or has served on speakers' bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen Pharmaceutics, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Sanofi and Takeda; he has received research support from Boehringer Ingelheim, Merck Sharp & Dohme Ltd and Novartis. J. R. has served on scientific advisory boards and received honoraria or consulting fees from companies involved in development of SGLT‐2 and DPP‐4 inhibitors, including AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Lexicon and Sanofi, and has received grants/research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and company, Janssen, Lexicon, Merck, Pfizer and Sanofi. C. M. serves or has served on advisory panels for AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly and Company, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, Mannkind, Medtronic, Merck Sharp & Dohme Ltd, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi and UCB and serves or has served on speakers' bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck Sharp and Dohme, Novartis, Novo Nordisk and Sanofi. University of Leuven has received research support for C. M. from Abbott, Eli Lilly and Company, Intrexon, Merck Sharp and Dohme Ltd, Novartis, Novo Nordisk, Roche Diagnostics and Sanofi.
Figures
References
-
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005‐2012. - PubMed
-
- American Diabetes Association . Standards of medical care in diabetes‐2016. Diabetes Care. 2016;39(suppl):S1‐S119.
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. - PubMed
-
- Rosenstock J, Hansen L, Zee P, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376‐383. - PubMed
-
- Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009‐2017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical