The quality of instruments to assess the process of shared decision making: A systematic review
- PMID: 29447193
- PMCID: PMC5813932
- DOI: 10.1371/journal.pone.0191747
The quality of instruments to assess the process of shared decision making: A systematic review
Abstract
Objective: To inventory instruments assessing the process of shared decision making and appraise their measurement quality, taking into account the methodological quality of their validation studies.
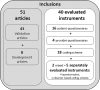
Methods: In a systematic review we searched seven databases (PubMed, Embase, Emcare, Cochrane, PsycINFO, Web of Science, Academic Search Premier) for studies investigating instruments measuring the process of shared decision making. Per identified instrument, we assessed the level of evidence separately for 10 measurement properties following a three-step procedure: 1) appraisal of the methodological quality using the COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) checklist, 2) appraisal of the psychometric quality of the measurement property using three possible quality scores, 3) best-evidence synthesis based on the number of studies, their methodological and psychometrical quality, and the direction and consistency of the results. The study protocol was registered at PROSPERO: CRD42015023397.
Results: We included 51 articles describing the development and/or evaluation of 40 shared decision-making process instruments: 16 patient questionnaires, 4 provider questionnaires, 18 coding schemes and 2 instruments measuring multiple perspectives. There is an overall lack of evidence for their measurement quality, either because validation is missing or methods are poor. The best-evidence synthesis indicated positive results for a major part of instruments for content validity (50%) and structural validity (53%) if these were evaluated, but negative results for a major part of instruments when inter-rater reliability (47%) and hypotheses testing (59%) were evaluated.
Conclusions: Due to the lack of evidence on measurement quality, the choice for the most appropriate instrument can best be based on the instrument's content and characteristics such as the perspective that they assess. We recommend refinement and validation of existing instruments, and the use of COSMIN-guidelines to help guarantee high-quality evaluations.
Conflict of interest statement
Figures
References
-
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–92. - PubMed
-
- Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172–9. doi: 10.1016/j.pec.2015.06.022 - DOI - PubMed
-
- Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. Jama. 1992;267(16):2221–6. - PubMed
-
- Wennberg JE, Barnes BA, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–24. - PubMed
-
- Couet N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18(4):542–61. doi: 10.1111/hex.12054 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical