Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes
- PMID: 29447265
- PMCID: PMC5814098
- DOI: 10.1371/journal.ppat.1006853
Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes
Abstract
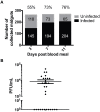
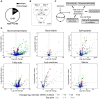
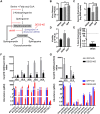
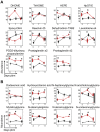
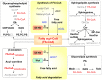
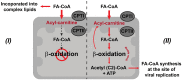
We describe the first comprehensive analysis of the midgut metabolome of Aedes aegypti, the primary mosquito vector for arboviruses such as dengue, Zika, chikungunya and yellow fever viruses. Transmission of these viruses depends on their ability to infect, replicate and disseminate from several tissues in the mosquito vector. The metabolic environments within these tissues play crucial roles in these processes. Since these viruses are enveloped, viral replication, assembly and release occur on cellular membranes primed through the manipulation of host metabolism. Interference with this virus infection-induced metabolic environment is detrimental to viral replication in human and mosquito cell culture models. Here we present the first insight into the metabolic environment induced during arbovirus replication in Aedes aegypti. Using high-resolution mass spectrometry, we have analyzed the temporal metabolic perturbations that occur following dengue virus infection of the midgut tissue. This is the primary site of infection and replication, preceding systemic viral dissemination and transmission. We identified metabolites that exhibited a dynamic-profile across early-, mid- and late-infection time points. We observed a marked increase in the lipid content. An increase in glycerophospholipids, sphingolipids and fatty acyls was coincident with the kinetics of viral replication. Elevation of glycerolipid levels suggested a diversion of resources during infection from energy storage to synthetic pathways. Elevated levels of acyl-carnitines were observed, signaling disruptions in mitochondrial function and possible diversion of energy production. A central hub in the sphingolipid pathway that influenced dihydroceramide to ceramide ratios was identified as critical for the virus life cycle. This study also resulted in the first reconstruction of the sphingolipid pathway in Aedes aegypti. Given conservation in the replication mechanisms of several flaviviruses transmitted by this vector, our results highlight biochemical choke points that could be targeted to disrupt transmission of multiple pathogens by these mosquitoes.
Conflict of interest statement
"The authors have declared that no competing interests exist."
Figures




References
-
- O’GOWER AK. The rate of digestion of human blood by certain species of mosquitoes. Australian Journal of Biological Sciences. 1955;9(1):125–9.
-
- Perera R, Kuhn RJ. Host metabolism and its contribution in Flavivirus biogenesis. In: Arboviruses: Molecular Biology, Evolution and Control in press. Gubler D, Vasilakis N, editors2015, In press.
-
- Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J Virol. 2014;88(9):4687–97. doi: 10.1128/JVI.00118-14 - DOI - PMC - PubMed
-
- Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, et al. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8(3):e1002584 doi: 10.1371/journal.ppat.1002584 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

