Super-resolution imaging identifies PARP1 and the Ku complex acting as DNA double-strand break sensors
- PMID: 29447383
- PMCID: PMC5909444
- DOI: 10.1093/nar/gky088
Super-resolution imaging identifies PARP1 and the Ku complex acting as DNA double-strand break sensors
Abstract
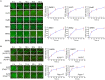
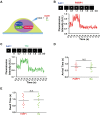
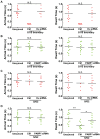
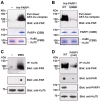
DNA double-strand breaks (DSBs) are fatal DNA lesions and activate a rapid DNA damage response. However, the earliest stage of DSB sensing remains elusive. Here, we report that PARP1 and the Ku70/80 complex localize to DNA lesions considerably earlier than other DSB sensors. Using super-resolved fluorescent particle tracking, we further examine the relocation kinetics of PARP1 and the Ku70/80 complex to a single DSB, and find that PARP1 and the Ku70/80 complex are recruited to the DSB almost at the same time. Notably, only the Ku70/80 complex occupies the DSB exclusively in the G1 phase; whereas PARP1 competes with the Ku70/80 complex at the DSB in the S/G2 phase. Moreover, in the S/G2 phase, PARP1 removes the Ku70/80 complex through its enzymatic activity, which is further confirmed by in vitro DSB-binding assays. Taken together, our results reveal PARP1 and the Ku70/80 complex as critical DSB sensors, and suggest that PARP1 may function as an important regulator of the Ku70/80 complex at the DSBs in the S/G2 phase.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

