Wilhelm His' lasting insights into hindbrain and cranial ganglia development and evolution
- PMID: 29447907
- PMCID: PMC6087689
- DOI: 10.1016/j.ydbio.2018.02.001
Wilhelm His' lasting insights into hindbrain and cranial ganglia development and evolution
Abstract
Wilhelm His (1831-1904) provided lasting insights into the development of the central and peripheral nervous system using innovative technologies such as the microtome, which he invented. 150 years after his resurrection of the classical germ layer theory of Wolff, von Baer and Remak, his description of the developmental origin of cranial and spinal ganglia from a distinct cell population, now known as the neural crest, has stood the test of time and more recently sparked tremendous advances regarding the molecular development of these important cells. In addition to his 1868 treatise on 'Zwischenstrang' (now neural crest), his work on the development of the human hindbrain published in 1890 provided novel ideas that more than 100 years later form the basis for penetrating molecular investigations of the regionalization of the hindbrain neural tube and of the migration and differentiation of its constituent neuron populations. In the first part of this review we briefly summarize the major discoveries of Wilhelm His and his impact on the field of embryology. In the second part we relate His' observations to current knowledge about the molecular underpinnings of hindbrain development and evolution. We conclude with the proposition, present already in rudimentary form in the writings of His, that a primordial spinal cord-like organization has been molecularly supplemented to generate hindbrain 'neomorphs' such as the cerebellum and the auditory and vestibular nuclei and their associated afferents and sensory organs.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

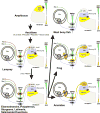
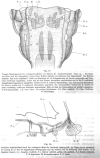

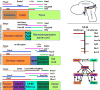



References
-
- Atwell WJ, Danchakoff V. A human embryo with seventeen pairs of somites. Carnegie Institution of Washington; 1930.
-
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. - PubMed
-
- Bonito M, Glover JC, Studer M. Hox genes and region-specific sensorimotor circuit formation in the hindbrain and spinal cord. Developmental Dynamics. 2013;242:1348–1368. - PubMed
Publication types
MeSH terms
Personal name as subject
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

