Recombinant FXIII (rFXIII-A2) Prophylaxis Prevents Bleeding and Allows for Surgery in Patients with Congenital FXIII A-Subunit Deficiency
- PMID: 29448295
- PMCID: PMC6260112
- DOI: 10.1055/s-0038-1624581
Recombinant FXIII (rFXIII-A2) Prophylaxis Prevents Bleeding and Allows for Surgery in Patients with Congenital FXIII A-Subunit Deficiency
Abstract
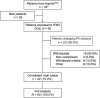
Recombinant factor XIII-A2 (rFXIII-A2) was developed for prophylaxis and treatment of bleeds in patients with congenital FXIII A-subunit deficiency. mentor™2 (NCT00978380), a multinational, open-label, single-arm, multiple-dosing extension to the pivotal mentor™1 trial, assessed long-term safety and efficacy of rFXIII-A2 prophylaxis in eligible patients (patients with severe [<0.05 IU/mL] congenital FXIII subunit A deficiency) aged ≥6 years. Patients received 35 IU/kg rFXIII-A2 (exact dosing) every 28 ± 2 days for ≥52 weeks. Primary endpoint was safety (adverse events including immunogenicity); secondary endpoints were rate of bleeds requiring FXIII treatment, haemostatic response after one 35 IU/kg rFXIII-A2 dose for breakthrough bleeds and withdrawals due to lack of rFXIII-A2 efficacy. Steady-state pharmacokinetic variables were also summarized. Elective surgery was permitted during the treatment period. Sixty patients were exposed to rFXIII-A2; their median age was 26.0 years (range: 7.0-77.0). rFXIII-A2 was well tolerated without any safety concerns. No non-neutralizing or neutralizing antibodies (inhibitors) against FXIII were detected. Mean annualized bleeding rate (ABR) was 0.043/patient-year. Mean spontaneous ABR was 0.011/patient-year. No patients withdrew due to lack of efficacy. Geometric mean FXIII trough level was 0.17 IU/mL. Geometric terminal half-life was 13.7 days. rFXIII-A2 prophylaxis provided sufficient haemostatic coverage for 12 minor surgeries without the need for additional FXIII therapy; eight procedures were performed within 7 days of the patient's last scheduled rFXIII-A2 dose, and four were performed 10 to 21 days after the last dose.
Schattauer GmbH Stuttgart.
Conflict of interest statement
M. Carcao has received research funding from Baxalta (now a part of Shire), Bayer HealthCare, Biogen, Novo Nordisk and Pfizer; additionally, he has received honoraria for advisory board participation and for speaking from Baxalta (now a part of Shire), Bayer HealthCare, Biogen, Biotest, CSL Behring, Novo Nordisk, Octapharma and Pfizer. C. Altisent has received honoraria or consultation fees from Baxalta (now a part of Shire), Bayer HealthCare, CSL Behring, Grifols, NovoNordisk, Octapharma, Pfizer and Sobi. G. Castaman has received honoraria for advisory board participation and for speaking from Baxalta (now a part of Shire), Bayer HealthCare, Biogen, CSL Behring, Novo Nordisk, Pfizer and Sobi, and has received research support from CSL Behring and Pfizer. K. Fukutake has received research grants from Baxalta (now a part of Shire), Bayer HealthCare, Biogen, CSL Behring, Japan Blood Products Organization, Kaketsuken, Novo Nordisk, Ortho Clinical Diagnostics and Pfizer, as well as honoraria and personal fees for participating in educational events, advisory board and publications from Abbott, Baxalta (now a part of Shire), Bayer HealthCare, Biogen, CSL Behring, Fujirebio Inc., Kaketsuken, LSI Medience, Novo Nordisk, Pfizer, Roche Diagnostics, Sekisui Medical, Siemens, SRL Inc. and Torii Pharmaceuticals outside the submitted work. B. Kerlin has served as an advisory board member for Bayer Healthcare US and Baxalta (now part of Shire), and has received research support from Novo Nordisk A/S and the CSL Behring foundation. C. Kessler has received grants from Baxalta (now a part of Shire), Bayer HealthCare, Biogen, Novo Nordisk, Octapharma, Pfizer and Roche. In addition, he has received honoraria and personal fees for participating in educational events and advisory boards from Baxalta (now a part of Shire), Bayer Healthcare, Biogen, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer and Roche. R. Lassila has received honoraria for advisory board participation from Baxalta (now a part of Shire), CSL Behring, Novo Nordisk, Octapharma and Pfizer. D. Nugent has no conflicts of interest to declare. J. Oldenburg has received reimbursement for attending symposia/congresses, and/or honoraria for speaking, and/or honoraria for consulting, and/or funds for research from Baxalta (now a part of Shire), Bayer HealthCare, Biogen Idec, Biotest, Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche and Swedish Orphan Biovitrum. M.-L. Garly is an employee of Novo Nordisk. A. Rosholm is an employee of Novo Nordisk and holds shares in Novo Nordisk. A. Inbal has no conflicts of interest to declare.
Figures
References
-
- Chung S I, Lewis M S, Folk J E. Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem. 1974;249(03):940–950. - PubMed
-
- Schwartz M L, Pizzo S V, Hill R L, McKee P A. Human factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973;248(04):1395–1407. - PubMed
-
- Muszbek L, Katona É. Diagnosis and Management of Congenital and Acquired FXIII Deficiencies. Semin Thromb Hemost. 2016;42(04):429–439. - PubMed
-
- Peyvandi F, Palla R, Menegatti M et al.Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. J Thromb Haemost. 2012;10(04):615–621. - PubMed
-
- Anwar R, Minford A, Gallivan L, Trinh C H, Markham A F. Delayed umbilical bleeding—a presenting feature for factor XIII deficiency: clinical features, genetics, and management. Pediatrics. 2002;109(02):E32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

