Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats
- PMID: 29449099
- PMCID: PMC5844852
- DOI: 10.1016/j.vaccine.2018.01.011
Cost of goods sold and total cost of delivery for oral and parenteral vaccine packaging formats
Abstract
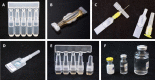
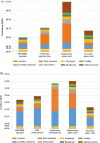
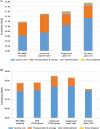
Despite limitations of glass packaging for vaccines, the industry has been slow to implement alternative formats. Polymer containers may address many of these limitations, such as breakage and delamination. However, the ability of polymer containers to achieve cost of goods sold (COGS) and total cost of delivery (TCOD) competitive with that of glass containers is unclear, especially for cost-sensitive low- and lower-middle-income countries. COGS and TCOD models for oral and parenteral vaccine packaging formats were developed based on information from subject matter experts, published literature, and Kenya's comprehensive multiyear plan for immunization. Rotavirus and inactivated poliovirus vaccines (IPV) were used as representative examples of oral and parenteral vaccines, respectively. Packaging technologies evaluated included glass vials, blow-fill-seal (BFS) containers, preformed polymer containers, and compact prefilled auto-disable (CPAD) devices in both BFS and preformed formats. For oral vaccine packaging, BFS multi-monodose (MMD) ampoules were the least expensive format, with a COGS of $0.12 per dose. In comparison, oral single-dose glass vials had a COGS of $0.40. BFS MMD ampoules had the lowest TCOD of oral vaccine containers at $1.19 per dose delivered, and ten-dose glass vials had a TCOD of $1.61 per dose delivered. For parenteral vaccines, the lowest COGS was achieved with ten-dose glass vials at $0.22 per dose. In contrast, preformed CPAD devices had the highest COGS at $0.60 per dose. Ten-dose glass vials achieved the lowest TCOD of the parenteral vaccine formats at $1.56 per dose delivered. Of the polymer containers for parenteral vaccines, BFS MMD ampoules achieved the lowest TCOD at $1.89 per dose delivered, whereas preformed CPAD devices remained the most expensive format, at $2.25 per dose delivered. Given their potential to address the limitations of glass and reduce COGS and TCOD, polymer containers deserve further consideration as alternative approaches for vaccine packaging.
Keywords: Cost of delivery; Manufacturing cost; Packaging; Poliovirus; Rotavirus; Vaccine.
Copyright © 2018 PATH. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Langille S.E. Particulate matter in injectable drug products. PDA J Pharm Sci Technol. 2013;67(3):186–200. - PubMed
-
- Bukofzer S., Ayres J., Chavez A., Devera M., Miller J., Ross D. Industry perspective on the medical risk of visible particles in injectable drug products. PDA J Pharm Sci Technol. 2015;69(1):123–139. - PubMed
-
- DeGrazio F, Paskiet D. The glass quandary. Contract Pharma, January 23, 2012 <http://www.contractpharma.com/issues/2012-01/view_features/the-glass-qua...> [accessed July 31, 2017].
-
- United Nations Children’s Fund (UNICEF). Vaccine wastage assessment: field assessment and observations from national stores and five selected states of India. UNICEF; 2010 <http://www.mofa.go.jp/mofaj/gaiko/oda/seisaku/kanmin/chusho_h24/pdfs/a20...> [accessed July 31, 2017].
-
- Making Medical Injections Safer project. The Incinerator Guidebook, Version 1. Seattle: PATH and John Snow Inc., March 2010 <http://www.path.org/publications/files/TS_mmis_incin_guide.pdf> [accessed November 2017].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

