Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: Confirming a non-pancreatic etiology
- PMID: 29449181
- PMCID: PMC5869737
- DOI: 10.1016/j.molmet.2017.12.011
Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: Confirming a non-pancreatic etiology
Abstract
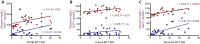
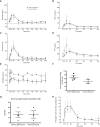
Objective: Postprandial hypoglycemia is an infrequent but disabling complication of Roux-en-Y gastric bypass (RYGB) surgery. Controversy still exists as to whether the postprandial hyperinsulinemia observed is due to inherent changes in pancreatic β-cell mass or function or to reversible alterations caused by RYGB anatomy. We aimed to determine if gastric feeding or reversal of RYGB would normalize postprandial glucose and hormone excursions in patients with symptomatic hypoglycemia.
Methods: We completed a prospective study of six patients with severe symptomatic RYGB hypoglycemia who underwent RYGB reversal. An additional subject without hypoglycemia who underwent RYGB reversal was also studied prospectively. Mixed meal tolerance testing (MTT) was done orally (RYGB anatomy), via gastrostomy tube in the excluded stomach in the setting of RYGB, and several months after RYGB reversal.
Results: All subjects reported symptomatic improvement of hypoglycemia after reversal of RYGB. Weight gain after reversal was moderate and variable. Postprandial glucose, insulin, and GLP-1 excursions were significantly diminished with gastric feeding and after reversal. Insulin secretion changed proportional to glucose levels and insulin clearance increased after reversal. Glucagon/insulin ratios were similar throughout study. We further compared the impact of modified sleeve gastrectomy reversal surgery to those with restoration of complete stomach and found no significant differences in weight regain or in postprandial glucose or hormone levels.
Conclusions: Reversal of RYGB is an effective treatment option for severe postprandial hypoglycemia. The pathophysiology of this disorder is primarily due to RYGB anatomy resulting in altered glucose, gut, and pancreatic hormone levels and decreased insulin clearance, rather than inherent β-cell hyperplasia or hyperfunction.
Keywords: Bariatric surgery; Gastric bypass reversal; Glucagon-like peptide 1; Hypoglycemia; Insulin; Roux en Y gastric bypass.
Published by Elsevier GmbH.
Figures






Comment in
-
Can reversal of RYGB also reverse hypoglycemia?Mol Metab. 2018 Mar;9:1-3. doi: 10.1016/j.molmet.2018.01.004. Epub 2018 Jan 12. Mol Metab. 2018. PMID: 29371089 Free PMC article. No abstract available.
Similar articles
-
Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia.J Clin Endocrinol Metab. 2013 Jul;98(7):E1208-12. doi: 10.1210/jc.2013-1151. Epub 2013 May 10. J Clin Endocrinol Metab. 2013. PMID: 23666968 Free PMC article.
-
Nutrient re-routing and altered gut-islet cell crosstalk may explain early relief of severe postprandial hypoglycaemia after reversal of Roux-en-Y gastric bypass.Diabet Med. 2017 Dec;34(12):1783-1787. doi: 10.1111/dme.13443. Diabet Med. 2017. PMID: 28782840
-
Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy.Gastroenterology. 2019 May;156(6):1627-1641.e1. doi: 10.1053/j.gastro.2019.01.262. Epub 2019 Feb 8. Gastroenterology. 2019. PMID: 30742833
-
Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass.Dan Med J. 2015 Apr;62(4):B5057. Dan Med J. 2015. PMID: 25872541 Review.
-
Postprandial hypoglycemia after gastric bypass surgery: from pathogenesis to diagnosis and treatment.Curr Opin Clin Nutr Metab Care. 2019 Jul;22(4):295-302. doi: 10.1097/MCO.0000000000000574. Curr Opin Clin Nutr Metab Care. 2019. PMID: 31082828 Free PMC article. Review.
Cited by
-
Weight-Independent Mechanisms of Glucose Control After Roux-en-Y Gastric Bypass.Front Endocrinol (Lausanne). 2018 Sep 10;9:530. doi: 10.3389/fendo.2018.00530. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30250454 Free PMC article. Review.
-
Hypoglycemia After Gastric Bypass Surgery: Current Concepts and Controversies.J Clin Endocrinol Metab. 2018 Aug 1;103(8):2815-2826. doi: 10.1210/jc.2018-00528. J Clin Endocrinol Metab. 2018. PMID: 30101281 Free PMC article. Review.
-
Postprandial hypoglycemia as a complication of bariatric and metabolic surgery: a comprehensive review of literature.Front Surg. 2024 Nov 1;11:1449012. doi: 10.3389/fsurg.2024.1449012. eCollection 2024. Front Surg. 2024. PMID: 39555226 Free PMC article. Review.
-
Changes in Symptoms and General Well-being After Reversal of Roux-en-Y Gastric Bypass: A Questionnaire Survey.Obes Surg. 2025 Jan;35(1):33-39. doi: 10.1007/s11695-024-07321-2. Epub 2024 May 29. Obes Surg. 2025. PMID: 38811426 Free PMC article.
-
The efficacy of GLP-1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review.Obesity (Silver Spring). 2023 Jan;31(1):20-30. doi: 10.1002/oby.23600. Epub 2022 Dec 10. Obesity (Silver Spring). 2023. PMID: 36502288 Free PMC article.
References
-
- Zimmet P.P., Alberti K.G.M.M.K. Surgery or medical therapy for obese patients with type 2 diabetes? New England Journal of Medicine. 2012;366(17):1635–1636. - PubMed
-
- Marsk R., Jonas E., Rasmussen F., Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. - PubMed
-
- Sarwar H., Chapman W.H., Pender J.R., Ivanescu A., Drake A.J., Pories W.J. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obesity Surgery. 2014;24(7):1120–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

