Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway
- PMID: 29449346
- PMCID: PMC5897745
- DOI: 10.1042/BSR20171546
Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway
Retraction in
-
Retraction: Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway.Biosci Rep. 2024 Feb 28;44(2):BSR-2017-1546_RET. doi: 10.1042/BSR-2017-1546_RET. Biosci Rep. 2024. PMID: 38414404 Free PMC article. No abstract available.
Abstract
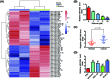
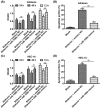
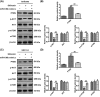
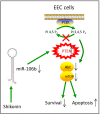
Shikonin, a natural naphthoquinone isolated from a traditional Chinese medicinal herb, which exerts anticancer effects in various cancers. However, the molecular mechanisms underlying the therapeutic effects of shikonin against endometrioid endometrial cancer (EEC) have not yet been fully elucidated. Herein, we investigated anticancer effects of shikonin on EEC cells and explored the underlying molecular mechanism. We observed that shikonin inhibits proliferation in human EEC cell lines in a dose-dependent manner. Moreover, shikonin-induced apoptosis was characterized by the up-regulation of the pro-apoptotic proteins cleaved-Caspase-3 and Bax, and the down-regulation of the anti-apoptotic protein Bcl-2. Microarray analyses demonstrated that shikonin induces many miRNAs' dysregulation, and miR-106b was one of the miRNAs being most significantly down-regulated. miR-106b was identified to exert procancer effect in various cancers, but in EEC remains unclear. We first confirmed that miR-106b is up-regulated in EEC tissues and cells, and knockdown of miR-106b suppresses proliferation and promotes apoptosis. Meanwhile, our results validated that the restored expression of miR-106b abrogates the antiproliferative and pro-apoptotic effects of shikonin. We also identified that miR-106b targets phosphatase and tensin homolog (PTEN), a tumor suppressor gene, which in turn modulates AKT/mTOR signaling pathway. Our findings indicated that shikonin inhibits proliferation and promotes apoptosis in human EEC cells by modulating the miR-106b/PTEN/AKT/mTOR signaling pathway, suggesting shikonin could act a potential therapeutic agent in the EEC treatment.
Keywords: Shikonin; ant-cancer; endometrioid endometrial cancer; miR-106b/PTEN/AKT/mTOR signaling pathway; microRNAs.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



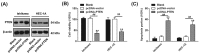
Similar articles
-
The Critical Role of PTEN/PI3K/AKT Signaling Pathway in Shikonin-Induced Apoptosis and Proliferation Inhibition of Chronic Myeloid Leukemia.Cell Physiol Biochem. 2018;47(3):981-993. doi: 10.1159/000490142. Epub 2018 May 24. Cell Physiol Biochem. 2018. PMID: 29843123
-
MicroRNA-106b promotes pituitary tumor cell proliferation and invasion through PI3K/AKT signaling pathway by targeting PTEN.Tumour Biol. 2016 Oct;37(10):13469-13477. doi: 10.1007/s13277-016-5155-2. Epub 2016 Jul 27. Tumour Biol. 2016. PMID: 27465551
-
Estrogen affects the negative feedback loop of PTENP1-miR200c to inhibit PTEN expression in the development of endometrioid endometrial carcinoma.Cell Death Dis. 2018 Dec 18;10(1):4. doi: 10.1038/s41419-018-1207-4. Cell Death Dis. 2018. PMID: 30584245 Free PMC article.
-
Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway.Leukemia. 2011 Jul;25(7):1064-79. doi: 10.1038/leu.2011.46. Epub 2011 Mar 25. Leukemia. 2011. PMID: 21436840 Review.
-
Role of signaling pathways in endometrial cancer.Mol Biol Rep. 2025 Apr 21;52(1):408. doi: 10.1007/s11033-025-10523-1. Mol Biol Rep. 2025. PMID: 40257522 Review.
Cited by
-
Isocorydine Exerts Anticancer Activity by Disrupting the Energy Metabolism and Filamentous Actin Structures of Oral Squamous Carcinoma Cells.Curr Issues Mol Biol. 2024 Jan 9;46(1):650-662. doi: 10.3390/cimb46010042. Curr Issues Mol Biol. 2024. PMID: 38248344 Free PMC article.
-
Radix Scrophulariae Extracts Exert Effect on Hyperthyroidism via MST1/Hippo Signaling Pathway.Chin J Integr Med. 2023 Nov;29(11):998-1006. doi: 10.1007/s11655-023-3744-7. Epub 2023 Sep 4. Chin J Integr Med. 2023. PMID: 37661231
-
Mechanisms and benefits of phytochemicals as an alternative therapeutic strategy in female cancers.Chin Herb Med. 2025 Apr 11;17(3):448-463. doi: 10.1016/j.chmed.2025.04.002. eCollection 2025 Jul. Chin Herb Med. 2025. PMID: 40734906 Free PMC article. Review.
-
Competitive glucose metabolism as a target to boost bladder cancer immunotherapy.Nat Rev Urol. 2020 Feb;17(2):77-106. doi: 10.1038/s41585-019-0263-6. Epub 2020 Jan 17. Nat Rev Urol. 2020. PMID: 31953517 Review.
-
Small Non-Coding-RNA in Gynecological Malignancies.Cancers (Basel). 2021 Mar 3;13(5):1085. doi: 10.3390/cancers13051085. Cancers (Basel). 2021. PMID: 33802524 Free PMC article. Review.
References
-
- Parker S.L., Tong T., Bolden S. and Wingo P.A. (1996) Cancer statistics, 1996. CA Cancer J. Clin. 46, 5–27 - PubMed
-
- Murayama-Hosokawa S., Oda K., Nakagawa S., Ishikawa S., Yamamoto S., Shoji K. et al. (2010) Genome-wide single-nucleotide polymorphism arrays in endometrial carcinomas associate extensive chromosomal instability with poor prognosis and unveil frequent chromosomal imbalances involved in the PI3-kinase pathway. Oncogene 29, 1897–1908 10.1038/onc.2009.474 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

