Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney
- PMID: 29449449
- PMCID: PMC5827607
- DOI: 10.1681/ASN.2017080890
Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney
Abstract
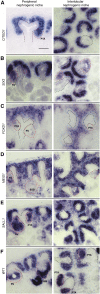
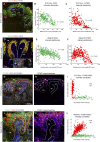
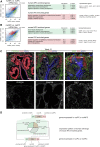
Cellular interactions among nephron, interstitial, and collecting duct progenitors drive mammalian kidney development. In mice, Six2+ nephron progenitor cells (NPCs) and Foxd1+ interstitial progenitor cells (IPCs) form largely distinct lineage compartments at the onset of metanephric kidney development. Here, we used the method for analyzing RNA following intracellular sorting (MARIS) approach, single-cell transcriptional profiling, in situ hybridization, and immunolabeling to characterize the presumptive NPC and IPC compartments of the developing human kidney. As in mice, each progenitor population adopts a stereotypical arrangement in the human nephron-forming niche: NPCs capped outgrowing ureteric branch tips, whereas IPCs were sandwiched between the NPCs and the renal capsule. Unlike mouse NPCs, human NPCs displayed a transcriptional profile that overlapped substantially with the IPC transcriptional profile, and key IPC determinants, including FOXD1, were readily detected within SIX2+ NPCs. Comparative gene expression profiling in human and mouse Six2/SIX2+ NPCs showed broad agreement between the species but also identified species-biased expression of some genes. Notably, some human NPC-enriched genes, including DAPL1 and COL9A2, are linked to human renal disease. We further explored the cellular diversity of mesenchymal cell types in the human nephrogenic niche through single-cell transcriptional profiling. Data analysis stratified NPCs into two main subpopulations and identified a third group of differentiating cells. These findings were confirmed by section in situ hybridization with novel human NPC markers predicted through the single-cell studies. This study provides a benchmark for the mesenchymal progenitors in the human nephrogenic niche and highlights species-variability in kidney developmental programs.
Keywords: human genetics; kidney development; nephron.
Copyright © 2018 by the American Society of Nephrology.
Figures






Comment in
-
The Era of Human Developmental Nephrology.J Am Soc Nephrol. 2018 Mar;29(3):705-706. doi: 10.1681/ASN.2017121280. Epub 2018 Feb 15. J Am Soc Nephrol. 2018. PMID: 29449450 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

