Conserved and Divergent Features of Human and Mouse Kidney Organogenesis
- PMID: 29449453
- PMCID: PMC5827606
- DOI: 10.1681/ASN.2017080887
Conserved and Divergent Features of Human and Mouse Kidney Organogenesis
Abstract
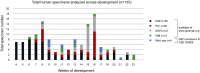
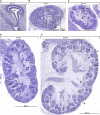
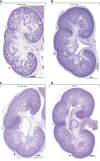
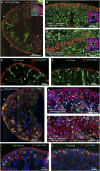
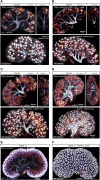
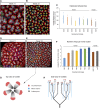
Human kidney function is underpinned by approximately 1,000,000 nephrons, although the number varies substantially, and low nephron number is linked to disease. Human kidney development initiates around 4 weeks of gestation and ends around 34-37 weeks of gestation. Over this period, a reiterative inductive process establishes the nephron complement. Studies have provided insightful anatomic descriptions of human kidney development, but the limited histologic views are not readily accessible to a broad audience. In this first paper in a series providing comprehensive insight into human kidney formation, we examined human kidney development in 135 anonymously donated human kidney specimens. We documented kidney development at a macroscopic and cellular level through histologic analysis, RNA in situ hybridization, immunofluorescence studies, and transcriptional profiling, contrasting human development (4-23 weeks) with mouse development at selected stages (embryonic day 15.5 and postnatal day 2). The high-resolution histologic interactive atlas of human kidney organogenesis generated can be viewed at the GUDMAP database (www.gudmap.org) together with three-dimensional reconstructions of key components of the data herein. At the anatomic level, human and mouse kidney development differ in timing, scale, and global features such as lobe formation and progenitor niche organization. The data also highlight differences in molecular and cellular features, including the expression and cellular distribution of anchor gene markers used to identify key cell types in mouse kidney studies. These data will facilitate and inform in vitro efforts to generate human kidney structures and comparative functional analyses across mammalian species.
Keywords: human genetics; kidney development; nephron.
Copyright © 2018 by the American Society of Nephrology.
Figures




Comment in
-
The Era of Human Developmental Nephrology.J Am Soc Nephrol. 2018 Mar;29(3):705-706. doi: 10.1681/ASN.2017121280. Epub 2018 Feb 15. J Am Soc Nephrol. 2018. PMID: 29449450 Free PMC article. No abstract available.
-
Evolution and Kidney Development: A Rosetta Stone for Nephrology.J Am Soc Nephrol. 2018 Mar;29(3):706-709. doi: 10.1681/ASN.2018010013. Epub 2018 Feb 15. J Am Soc Nephrol. 2018. PMID: 29449452 Free PMC article. No abstract available.
References
-
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M: Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

