Enantioselective C(sp3)‒H bond activation by chiral transition metal catalysts
- PMID: 29449462
- PMCID: PMC5862070
- DOI: 10.1126/science.aao4798
Enantioselective C(sp3)‒H bond activation by chiral transition metal catalysts
Abstract
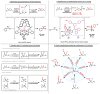
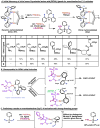
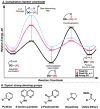
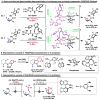
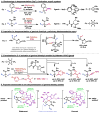
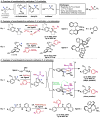
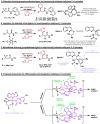
Organic molecules are rich in carbon-hydrogen bonds; consequently, the transformation of C-H bonds to new functionalities (such as C-C, C-N, and C-O bonds) has garnered much attention by the synthetic chemistry community. The utility of C-H activation in organic synthesis, however, cannot be fully realized until chemists achieve stereocontrol in the modification of C-H bonds. This Review highlights recent efforts to enantioselectively functionalize C(sp3)-H bonds via transition metal catalysis, with an emphasis on key principles for both the development of chiral ligand scaffolds that can accelerate metalation of C(sp3)-H bonds and stereomodels for asymmetric metalation of prochiral C-H bonds by these catalysts.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
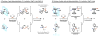
References
-
- Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Selective intermolecular carbon-hydrogen activation by synthetic metal complexes in homogeneous solution. Acc Chem Res. 1995;28:154–162.
-
- Carey JS, Laffan D, Thomson C, Williams MT. Analysis of the reactions used for the preparation of drug candidate molecules. Org Biomol Chem. 2006;4:2337–2347. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

