Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle
- PMID: 29449621
- PMCID: PMC5814568
- DOI: 10.1038/s41598-018-20409-x
Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle
Abstract
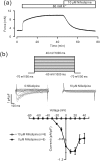
Because of the serious side effects of the currently used bronchodilators, new compounds with similar functions must be developed. We screened several herbs and found that Polygonum aviculare L. contains ingredients that inhibit the precontraction of mouse and human airway smooth muscle (ASM). High K+-induced precontraction in ASM was completely inhibited by nifedipine, a selective blocker of L-type voltage-dependent Ca2+ channels (LVDCCs). However, nifedipine only partially reduced the precontraction induced by acetylcholine chloride (ACH). Additionally, the ACH-induced precontraction was partly reduced by pyrazole-3 (Pyr3), a selective blocker of TRPC3 and stromal interaction molecule (STIM)/Orai channels. These channel-mediated currents were inhibited by the compounds present in P. aviculare extracts, suggesting that this inhibition was mediated by LVDCCs, TRPC3 and/or STIM/Orai channels. Moreover, these channel-mediated currents were inhibited by quercetin, which is present in P. aviculare extracts. Furthermore, quercetin inhibited ACH-induced precontraction in ASM. Overall, our data indicate that the ethyl acetate fraction of P. aviculare and quercetin can inhibit Ca2+-permeant LVDCCs, TRPC3 and STIM/Orai channels, which inhibits the precontraction of ASM. These findings suggest that P. aviculare could be used to develop new bronchodilators to treat obstructive lung diseases such as asthma and chronic obstructive pulmonary disease.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

