Reactive Oxygen Species: A Key Constituent in Cancer Survival
- PMID: 29449774
- PMCID: PMC5808965
- DOI: 10.1177/1177271918755391
Reactive Oxygen Species: A Key Constituent in Cancer Survival
Abstract
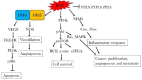
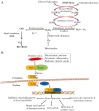
Background: Cancer is one of the major heterogeneous disease with high morbidity and mortality with poor prognosis. Elevated levels of reactive oxygen species (ROS), alteration in redox balance, and deregulated redox signaling are common hallmarks of cancer progression and resistance to treatment. Mitochondria contribute mainly in the generation of ROS during oxidative phosphorylation. Elevated levels of ROS have been detected in cancers cells due to high metabolic activity, cellular signaling, peroxisomal activity, mitochondrial dysfunction, activation of oncogene, and increased enzymatic activity of oxidases, cyclooxygenases, lipoxygenases, and thymidine phosphorylases. Cells maintain intracellular homeostasis by developing an immense antioxidant system including catalase, superoxide dismutase, and glutathione peroxidase. Besides these enzymes exist an important antioxidant glutathione and transcription factor Nrf2 which contribute in balancing oxidative stress. Reactive oxygen species-mediated signaling pathways activate pro-oncogenic signaling which eases in cancer progression, angiogenesis, and survival. Concomitantly, to maintain ROS homeostasis and evade cancer cell death, an increased level of antioxidant capacity is associated with cancer cells.
Conclusions: This review focuses the role of ROS in cancer survival pathways and importance of targeting the ROS signal involved in cancer development, which is a new strategy in cancer treatment.
Keywords: MMPs; Nrf2; ROS; cancer survival.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Levine AS, Sun L, Tan R, et al. The oxidative DNA damage response: a review of research undertaken with Tsinghua and Xiangya students at the University of Pittsburgh. Sci China Life Sci. 2017;60:1077–1080. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

