Atrial Natriuretic Peptide Acts as a Neuroprotective Agent in in Vitro Models of Parkinson's Disease via Up-regulation of the Wnt/β-Catenin Pathway
- PMID: 29449807
- PMCID: PMC5799264
- DOI: 10.3389/fnagi.2018.00020
Atrial Natriuretic Peptide Acts as a Neuroprotective Agent in in Vitro Models of Parkinson's Disease via Up-regulation of the Wnt/β-Catenin Pathway
Abstract
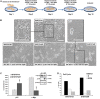
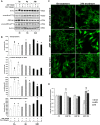
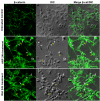
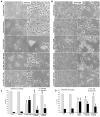
In the last decades increasing evidence indicated a crucial role of the Wnt/β-catenin signaling in development of midbrain dopaminergic (mDA) neurons. Recently dysregulation of this pathway has been proposed as a novel pathomechanism leading to Parkinson's disease (PD) and some of the molecules participating to the signaling have been evaluated as potential therapeutic targets for PD. Atrial natriuretic peptide (ANP) is a cardiac-derived hormone having a critical role in cardiovascular homeostasis. ANP and its receptors (NPRs) are widely expressed in mammalian central nervous system (CNS) where they could be implicated in the regulation of neural development, synaptic transmission and information processing, as well as in neuroprotection. Until now, the effects of ANP in the CNS have been mainly ascribed to the binding and activation of NPRs. We have previously demonstrated that ANP affects the Wnt/β-catenin signaling in colorectal cancer cells through a Frizzled receptor-mediated mechanism. The purpose of this study was to investigate if ANP is able to exert neuroprotective effect on two in vitro models of PD, and if this effect could be related to activation of the Wnt/β-catenin signaling. As cellular models of DA neurons, we used the proliferating or RA-differentiated human neuroblastoma cell line SH-SY5Y. In both DA neuron-like cultures, ANP is able to positively affect the Wnt/β-catenin signaling, by inducing β-catenin stabilization and nuclear translocation. Importantly, activation of the Wnt pathway by ANP exerts neuroprotective effect when these two cellular systems were subjected to neurotoxic insult (6-OHDA) for mimicking the neurodegeneration of PD. Our data support the relevance of exogenous ANP as an innovative therapeutic molecule for midbrain, and more in general for brain diseases for which aberrant Wnt signaling seems to be involved.
Keywords: Parkinson’s disease; Wnt/β-catenin pathway; atrial natriuretic peptide; neurodegeneration; neuroprotection.
Figures




Similar articles
-
Natriuretic peptides are neuroprotective on in vitro models of PD and promote dopaminergic differentiation of hiPSCs-derived neurons via the Wnt/β-catenin signaling.Cell Death Discov. 2021 Nov 1;7(1):330. doi: 10.1038/s41420-021-00723-6. Cell Death Discov. 2021. PMID: 34725335 Free PMC article.
-
Anti-proliferative effect of atrial natriuretic peptide on colorectal cancer cells: evidence for an Akt-mediated cross-talk between NHE-1 activity and Wnt/β-catenin signaling.Biochim Biophys Acta. 2012 Jun;1822(6):1004-18. doi: 10.1016/j.bbadis.2012.02.016. Epub 2012 Feb 25. Biochim Biophys Acta. 2012. PMID: 22387884
-
Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson's disease model.Biochem Pharmacol. 2017 Sep 15;140:115-123. doi: 10.1016/j.bcp.2017.05.017. Epub 2017 May 25. Biochem Pharmacol. 2017. PMID: 28551099
-
Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain.Front Aging Neurosci. 2018 Feb 12;10:12. doi: 10.3389/fnagi.2018.00012. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29483868 Free PMC article. Review.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
Cited by
-
Wnt1 silencing enhances neurotoxicity induced by paraquat and maneb in SH-SY5Y cells.Exp Ther Med. 2019 Nov;18(5):3643-3649. doi: 10.3892/etm.2019.7963. Epub 2019 Aug 30. Exp Ther Med. 2019. PMID: 31602242 Free PMC article.
-
Atrial Natriuretic Peptide Promotes Neurite Outgrowth and Survival of Cochlear Spiral Ganglion Neurons in vitro Through NPR-A/cGMP/PKG Signaling.Front Cell Dev Biol. 2021 Jun 23;9:681421. doi: 10.3389/fcell.2021.681421. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34268307 Free PMC article.
-
The recent progress of peptide regulators for the Wnt/β-catenin signaling pathway.Front Med (Lausanne). 2023 Jun 16;10:1164656. doi: 10.3389/fmed.2023.1164656. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37396899 Free PMC article. Review.
-
The Wnt/β-catenin signaling: a multifunctional target for neuroprotective and regenerative strategies in Parkinson's disease.Neural Regen Res. 2023 Feb;18(2):306-308. doi: 10.4103/1673-5374.343908. Neural Regen Res. 2023. PMID: 35900408 Free PMC article. No abstract available.
-
Nature-derived compounds modulating Wnt/ β -catenin pathway: a preventive and therapeutic opportunity in neoplastic diseases.Acta Pharm Sin B. 2020 Oct;10(10):1814-1834. doi: 10.1016/j.apsb.2019.12.019. Epub 2020 Jan 7. Acta Pharm Sin B. 2020. PMID: 33163337 Free PMC article. Review.
References
-
- Canet-Aviles R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., et al. (2004). The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U.S.A. 101 9103–9108. 10.1073/pnas.0402959101 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

