Putative roles of mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family proteins and c-Jun N-terminal Kinases in ischemic stroke associated apoptosis
- PMID: 29450141
- PMCID: PMC5802046
- DOI: 10.1016/j.biopen.2017.02.002
Putative roles of mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family proteins and c-Jun N-terminal Kinases in ischemic stroke associated apoptosis
Abstract
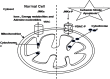
There is a constant need for better stroke treatments. Neurons at the periphery of an ischemic stroke affected brain tissue remains metabolically active for several hours or days after stroke onset. They later undergo mitochondrion-mediated apoptosis. It has been found that inhibiting apoptosis in the peripheral ischemic neurons could be very effective in the prevention of stroke progression. During stroke associated apoptosis, cytosolic c-Jun N-terminal Kinases (JNKs) and Bcl-2 family proteins translocate towards mitochondria and promote cytochrome c release by interacting with the outer mitochondrion membrane associated proteins. This review provides an overview of the plausible interactions of the outer mitochondrial membrane Voltage Dependent Anion Channel, Bcl-2 family proteins and JNKs in cytochrome c release in the peripheral ischemic stroke associated apoptotic neurons. The review ends with a note on designing new anti-stroke treatments.
Keywords: Bif-1, Bax interacting factor-1; Ischemic penumbra; JNKs, c-Jun N-terminal Kinases; MPT pore, Mitochondrial Permeability Transition pore; Mitochondrion-mediated apoptosis; VDAC, Voltage Dependent Anion Channel; Voltage-Dependent Anion Channel; c-Jun N-terminal Kinases.
Figures



References
-
- Broughton B.R., Reutens D.C., Sobey C.G. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009 May 1;40(5):e331–e339. - PubMed
-
- Coffey E.T. Nuclear and cytosolic JNK signalling in neurons. Nat. Rev. Neurosci. 2014;15(5):285–299. - PubMed
-
- Sims N.R., Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. BBA Mol. Basis Dis. 2010;1802(1):80–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

