Monitoring long-term oral corticosteroids
- PMID: 29450303
- PMCID: PMC5699140
- DOI: 10.1136/bmjoq-2017-000209
Monitoring long-term oral corticosteroids
Abstract
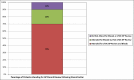
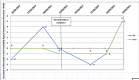
Corticosteroids are synthetic analogues of human hormones normally produced by the adrenal cortex. They have both glucocorticoid and mineralocorticoid properties. The glucocortoid components are anti-inflammatory, immunosuppressive, anti-proliferative and vasoconstrictive. They influence the metabolism of carbohydrate and protein, in addition to playing a key role in the body's stress response. Mineralocorticoid's main significance is in the balance of salt and water concentrations. Due to the combination of these effects, corticosteroids can cause many adverse effects. Oral corticosteroids are absorbed systemically and are therefore more likely to cause adverse effects than topical or inhaled corticosteroids. Furthermore, it is assumed that greater duration of treatment will lead to a greater number of adverse effects, and therefore the most at risk group are those taking high dose, long-term oral corticosteroids (LTOC). High dose is defined as a prescription of >5 mg oral prednisolone and long term as duration of treatment >1 month (based on National Institute for Health and Care Excellence guidance for patient's 'at risk' of systemic side effects). Parameters to be monitored in primary care include weight, blood pressure, triglycerides, glucose and urea and electrolytes. From clinical experience within the general practice setting, the authors propose that these patients do not receive adequate baseline monitoring before starting corticosteroids nor are these markers monitored consistently thereafter. This project intended to evidence this claim, evaluate the adverse effect profile and improve monitoring in this patient group. The initial audit of 22 patients, within a single general practice, detected at least one documented adverse effect in 64% of patients, while 41% reported more than one adverse effect. 45% had recorded weight gain, 18% had recorded osteoporosis, 18% had at least one recorded cataract, 14% had recorded Hypertension, 14% had recorded diabetes mellitus, 9% had recorded dyspepsia and 5% had a recorded psychiatric complaint. All of these recorded conditions were either directly attributed to steroid medication or occurred since LTOC were prescribed. The aim of this project was to increase the percentage of patients on LTOC with complete baseline monitoring to 100%. 'Baseline monitoring' was defined as a measurement taken within the previous 5 years. Although somewhat arbitrary, 5 years was felt to be the maximum timeframe in which monitoring would still be relevant for comparison following introduction of LTOC. Quality improvement methodology was used throughout this project with multiple PDSA (Plan, Study, Do and Act) cycles. Through this, a monitoring system and protocol for patients taking LTOC was developed. As a result of this project, five adverse effects were detected in five different patients. These included two cases of secondary hypertension, one case of diabetes mellitus, one cataract and one case of adrenal insufficiency. 12 out of 20 patients achieved complete baseline monitoring. While this study did not fully achieve its aim, the aim was deliberately ambitious. As not all patients in this study attended for monitoring, a figure of 100% was impossible to achieve. The remaining 'incompletely monitored patients' had some but not all parameters measured. The creation of a staff protocol and increased clinical experience will ensure that complete monitoring takes place in the future. In conclusion, this project has shown that adverse effects from LTOC are prevalent in a single general practice population. It is also shown that monitoring for LTOC adverse effects is inadequate but can be improved relatively easily as skills and competencies from other medication monitoring systems already exist within healthcare settings and are immediately transferable.
Keywords: adverse events, epidemiology and detection; healthcare quality improvement; primary care.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- NICE. Corticosteroids - oral. https://cks.nice.org.uk/corticosteroids-oral (accessed Apr 2017).
-
- Allen-Ramey FC, Nelsen LM, Leader JB, et al. . Electronic health record-based assessment of oral corticosteroid use in a population of primary care patients with asthma: an observational study. Allergy, Asthma and Clin Immunol 2013;9:27 doi:10.1186/1710-1492-9-27 - DOI - PMC - PubMed
-
- Sullivan PW, Ghushchyan VH, Globe G, et al. . Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol 2017:pii: S0091-6749(17)30680-2 (accessed Apr 2017). doi:10.1016/j.jaci.2017.04.009 - DOI - PubMed
-
- Broder MS, Sarsour K, Chang E, et al. . Corticosteroid-related adverse events in patients with giant cell arteritis: a claims-based analysis. Semin Arthritis Rheum 2016;46:246–52. doi:10.1016/j.semarthrit.2016.05.009 - DOI - PubMed
-
- Perdoncini-Roux A, Blanchon T, Hanslik T, et al. . [General practitioners' perception of the impact of corticosteroid-induced adverse events]. Rev Epidemiol Sante Publique 2009;57:93–7. doi:10.1016/j.respe.2008.12.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
