Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial
- PMID: 29450452
- PMCID: PMC5885262
- DOI: 10.1001/jamaoncol.2017.4996
Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial
Erratum in
-
Errors in Author Affiliations.JAMA Oncol. 2018 Apr 1;4(4):590. doi: 10.1001/jamaoncol.2018.1047. JAMA Oncol. 2018. PMID: 29677377 Free PMC article. No abstract available.
Abstract
Importance: Patients with soft tissue sarcoma are at risk for local recurrence and distant metastases despite optimal local treatment. Preoperative anthracycline plus ifosfamide chemotherapy improves outcome in common histological subtypes.
Objective: To analyze whether the previously reported improvement in local progression-free survival by adding regional hyperthermia to neoadjuvant chemotherapy translates into improved survival.
Design, setting, and participants: Open-label, phase 3 randomized clinical trial to evaluate the efficacy and toxic effects of neoadjuvant chemotherapy plus regional hyperthermia. Adult patients (age ≥18 years) with localized soft tissue sarcoma (tumor ≥5 cm, French Federation Nationale des Centers de Lutte Contre le Cancer [FNCLCC] grade 2 or 3, deep) were accrued across 9 centers (6, Germany; 1, Norway; 1, Austria; 1, United States) from July 1997 to November 2006. Follow-up ended December 2014.
Interventions: After stratification for tumor presentation and site, patients were randomly assigned to either neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide alone, or combined with regional hyperthermia.
Main outcomes and measures: The primary end point was local progression-free survival. Secondary end points included treatment safety and survival, with survival defined from date of randomization to death due to disease or treatment. Patients lost to follow-up were censored at the date of their last follow-up.
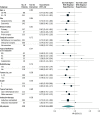
Results: A total of 341 patients were randomized, and 329 (median [range] age, 51 [18-70] years; 147 women, 182 men) were eligible for the intention-to-treat analysis. By December 2014, 220 patients (67%; 95% CI, 62%-72%) had experienced disease relapse, and 188 (57%; 95% CI, 52%-62%) had died. Median follow-up was 11.3 years. Compared with neoadjuvant chemotherapy alone, adding regional hyperthermia improved local progression-free survival (hazard ratio [HR], 0.65; 95% CI, 0.49-0.86; P = .002). Patients randomized to chemotherapy plus hyperthermia had prolonged survival rates compared with those randomized to neoadjuvant chemotherapy alone (HR, 0.73; 95% CI, 0.54-0.98; P = .04) with 5-year survival of 62.7% (95% CI, 55.2%-70.1%) vs 51.3% (95% CI, 43.7%-59.0%), respectively, and 10-year survival of 52.6% (95% CI, 44.7%-60.6%) vs 42.7% (95% CI, 35.0%-50.4%).
Conclusions and relevance: Among patients with localized high-risk soft tissue sarcoma the addition of regional hyperthermia to neoadjuvant chemotherapy resulted in increased survival, as well as local progression-free survival. For patients who are candidates for neoadjuvant treatment, adding regional hyperthermia may be warranted.
Trial registration: clinicaltrials.gov Identifier: NCT00003052.
Conflict of interest statement
Figures
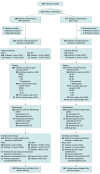

Comment in
-
Technological Advances, Biologic Rationales, and the Associated Success of Chemotherapy With Hyperthermia in Improved Outcomes in Patients With Sarcoma.JAMA Oncol. 2018 Apr 1;4(4):493-494. doi: 10.1001/jamaoncol.2017.4941. JAMA Oncol. 2018. PMID: 29450459 No abstract available.
-
Sarcoma: Local hyperthermia improves survival.Nat Rev Clin Oncol. 2018 May;15(5):266. doi: 10.1038/nrclinonc.2018.38. Epub 2018 Mar 6. Nat Rev Clin Oncol. 2018. PMID: 29508856 No abstract available.
-
[Locoregional hyperthermia improves disease-specific overall survival in combination with neoadjuvant chemotherapy in high-risk soft tissue sarcomas].Strahlenther Onkol. 2018 Aug;194(8):787-789. doi: 10.1007/s00066-018-1322-2. Strahlenther Onkol. 2018. PMID: 29882096 German. No abstract available.
-
Regional Hyperthermia With Neoadjuvant Chemotherapy for Treatment of Soft Tissue Sarcoma-Reply.JAMA Oncol. 2019 Jan 1;5(1):114. doi: 10.1001/jamaoncol.2018.5293. JAMA Oncol. 2019. PMID: 30489599 No abstract available.
-
Regional Hyperthermia With Neoadjuvant Chemotherapy for Treatment of Soft Tissue Sarcoma.JAMA Oncol. 2019 Jan 1;5(1):113-114. doi: 10.1001/jamaoncol.2018.5296. JAMA Oncol. 2019. PMID: 30489603 No abstract available.
-
Regional Hyperthermia With Neoadjuvant Chemotherapy for Treatment of Soft Tissue Sarcoma.JAMA Oncol. 2019 Jan 1;5(1):112-113. doi: 10.1001/jamaoncol.2018.5287. JAMA Oncol. 2019. PMID: 30489612 No abstract available.
Similar articles
-
Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study.Lancet Oncol. 2010 Jun;11(6):561-70. doi: 10.1016/S1470-2045(10)70071-1. Epub 2010 Apr 29. Lancet Oncol. 2010. PMID: 20434400 Free PMC article. Clinical Trial.
-
Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial.Lancet Oncol. 2017 Jun;18(6):812-822. doi: 10.1016/S1470-2045(17)30334-0. Epub 2017 May 9. Lancet Oncol. 2017. PMID: 28499583 Clinical Trial.
-
Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial.Lancet Oncol. 2014 Apr;15(4):415-23. doi: 10.1016/S1470-2045(14)70063-4. Epub 2014 Mar 5. Lancet Oncol. 2014. PMID: 24618336 Clinical Trial.
-
[Systemic therapy and hyperthermia for locally advanced soft tissue sarcoma].Chirurg. 2014 May;85(5):398-403. doi: 10.1007/s00104-013-2687-5. Chirurg. 2014. PMID: 24740176 Review. German.
-
Current trials and new aspects in soft tissue sarcoma of adults.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S4-8. doi: 10.1007/s00280-002-0445-3. Epub 2002 Apr 16. Cancer Chemother Pharmacol. 2002. PMID: 12042982 Review.
Cited by
-
Prediction tools for the personalized management of soft-tissue sarcomas of the extremity.Bone Joint J. 2022 Sep;104-B(9):1011-1016. doi: 10.1302/0301-620X.104B9.BJJ-2022-0647. Bone Joint J. 2022. PMID: 36047022 Free PMC article. Review.
-
Spatiotemporal control of engineered bacteria to express interferon-γ by focused ultrasound for tumor immunotherapy.Nat Commun. 2022 Aug 2;13(1):4468. doi: 10.1038/s41467-022-31932-x. Nat Commun. 2022. PMID: 35918309 Free PMC article.
-
Thermal distribution, physiological effects and toxicities of extracorporeally induced whole-body hyperthermia in a pig model.Physiol Rep. 2020 Feb;8(4):e14366. doi: 10.14814/phy2.14366. Physiol Rep. 2020. PMID: 32097540 Free PMC article.
-
LDH and hemoglobin outperform systemic inflammatory indices as prognostic factors in patients with soft tissue sarcoma undergoing neoadjuvant treatment.BMC Cancer. 2025 Mar 18;25(1):496. doi: 10.1186/s12885-025-13889-4. BMC Cancer. 2025. PMID: 40102864 Free PMC article.
-
The Immune Contexture of Liposarcoma and Its Clinical Implications.Cancers (Basel). 2022 Sep 21;14(19):4578. doi: 10.3390/cancers14194578. Cancers (Basel). 2022. PMID: 36230502 Free PMC article. Review.
References
-
- American Cancer Society . Cancer Facts and Figures 2017. 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-.... Accessed July 13, 2017.
-
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701-711. - PubMed
-
- Callegaro D, Miceli R, Bonvalot S, et al. . Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671-680. - PubMed
-
- Gronchi A, Miceli R, Shurell E, et al. . Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649-1655. - PubMed
-
- Group ESESNW; ESMO/European Sarcoma Network Working Group . Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii102-iii112. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

