Pediatric-Inspired Treatment Regimens for Adolescents and Young Adults With Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Review
- PMID: 29450465
- PMCID: PMC7534395
- DOI: 10.1001/jamaoncol.2017.5305
Pediatric-Inspired Treatment Regimens for Adolescents and Young Adults With Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Review
Abstract
Importance: The incidence of acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) in adolescent and young adult (AYA) patients (age range, 15-39 years) in the United States is increasing at a greater rate than in younger or older persons. Their optimal treatment has been increasingly debated as pediatric regimens have become more widely used in the age group. This review compares the basic features of pediatric and adult chemotherapy regimens for ALL and LBL, recognizes and describes the challenges of the pediatric regimen, and suggests strategies to facilitate its adoption for AYAs with ALL and LBL.
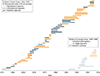
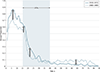
Observations: All but 2 of 25 published comparisons of outcomes with pediatric and adult regimens for ALL and LBL in AYAs and 1 meta-analysis favor the pediatric regimen. After more than a half-century of clinical trials of the pediatric regimens, including at least 160 phase 3 trials in the United States, the pediatric regimens have become far more complex than most adult regimens. Asparaginase, a critical component of the pediatric regimens, is more difficult to administer to AYAs (and older patients) but nonetheless has a favorable benefit to toxicity ratio for AYAs. A dramatic reduction in outcome of ALL and LBL during the AYA years (the "survival cliff") is coincident with similar reductions in proportions of AYAs referred to academic centers and enrolled on clinical trials (the "accrual cliff" and "referral cliff").
Conclusions and relevance: The accumulating data increasingly support treating AYAs with ALL and LBL with a pediatric-inspired regimen or an approved institutional or national clinical trial tailored for this patient group. A need to develop clinical trials specifically for AYAs and to encourage their participation is paramount, with a goal to improve both the quantity and quality of survival.
Figures
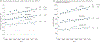
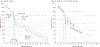
References
-
- Alliance for Clinical Trials in Oncology. A Phase 3 Trial to Evaluate the Efficacy of the Addition of Inotuzumab Ozogamicin to Frontline Therapy in Young Adults (Ages 18 to 39 Years) With Newly Diagnosed Precursor B-cell ALL. Alliance A041501 https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.... Accessed September 5, 2017.
-
- Stock W, La M, Sanford B, et al.; Children’s Cancer Group; Cancer and Leukemia Group B Studies. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? a comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5): 1646–1654. - PMC - PubMed
-
- Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute. SEER*Stat Database: Incidence SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment> Linked To County Attributes Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
-
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. [published correction appears in Stat Med. 2001;20(4):655]. Stat Med. 2000;19(3):335–351. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

