Access to the CNS: Biomarker Strategies for Dopaminergic Treatments
- PMID: 29450650
- PMCID: PMC5814527
- DOI: 10.1007/s11095-017-2333-x
Access to the CNS: Biomarker Strategies for Dopaminergic Treatments
Erratum in
-
Correction to: Access to the CNS: Biomarker Strategies for DopaminergicTreatments.Pharm Res. 2018 Mar 19;35(5):102. doi: 10.1007/s11095-018-2388-3. Pharm Res. 2018. PMID: 29556786 Free PMC article.
Abstract
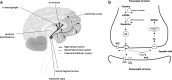
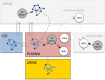
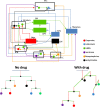
Despite substantial research carried out over the last decades, it remains difficult to understand the wide range of pharmacological effects of dopaminergic agents. The dopaminergic system is involved in several neurological disorders, such as Parkinson's disease and schizophrenia. This complex system features multiple pathways implicated in emotion and cognition, psychomotor functions and endocrine control through activation of G protein-coupled dopamine receptors. This review focuses on the system-wide effects of dopaminergic agents on the multiple biochemical and endocrine pathways, in particular the biomarkers (i.e., indicators of a pharmacological process) that reflect these effects. Dopaminergic treatments developed over the last decades were found to be associated with numerous biochemical pathways in the brain, including the norepinephrine and the kynurenine pathway. Additionally, they have shown to affect peripheral systems, for example the hypothalamus-pituitary-adrenal (HPA) axis. Dopaminergic agents thus have a complex and broad pharmacological profile, rendering drug development challenging. Considering the complex system-wide pharmacological profile of dopaminergic agents, this review underlines the needs for systems pharmacology studies that include: i) proteomics and metabolomics analysis; ii) longitudinal data evaluation and mathematical modeling; iii) pharmacokinetics-based interpretation of drug effects; iv) simultaneous biomarker evaluation in the brain, the cerebrospinal fluid (CSF) and plasma; and v) specific attention to condition-dependent (e.g., disease) pharmacology. Such approach is considered essential to increase our understanding of central nervous system (CNS) drug effects and substantially improve CNS drug development.
Keywords: CNS drug development; biomarkers; dopaminergic agents; systems pharmacology.
Figures

Similar articles
-
Therapeutic efficacy of atypical antipsychotic drugs by targeting multiple stress-related metabolic pathways.Transl Psychiatry. 2017 May 16;7(5):e1130. doi: 10.1038/tp.2017.94. Transl Psychiatry. 2017. PMID: 28509906 Free PMC article.
-
[Central dopaminergic receptors (Part II): pathophysiological and therapeutic considerations].Rev Neurol (Paris). 2004 Oct;160(10):986-92. doi: 10.1016/s0035-3787(04)71137-6. Rev Neurol (Paris). 2004. PMID: 15492728 Review. French.
-
Dopamine receptor pharmacology: interactions with serotonin receptors and significance for the aetiology and treatment of schizophrenia.CNS Neurol Disord Drug Targets. 2006 Feb;5(1):3-23. doi: 10.2174/187152706784111614. CNS Neurol Disord Drug Targets. 2006. PMID: 16613551 Review.
-
Addictions and stress: clues for cocaine pharmacotherapies.Curr Pharm Des. 2013;19(40):7065-80. doi: 10.2174/13816128113199990610. Curr Pharm Des. 2013. PMID: 23574443 Review.
-
Pharmacological strategies against glucocorticoid-mediated brain damage during chronic disorders.Recent Pat CNS Drug Discov. 2011 Sep 1;6(3):196-204. doi: 10.2174/157488911796958020. Recent Pat CNS Drug Discov. 2011. PMID: 21834781 Review.
Cited by
-
Abnormalities of Resting-State Electroencephalographic Microstate in Rapid Eye Movement Sleep Behavior Disorder.Front Hum Neurosci. 2021 Oct 22;15:728405. doi: 10.3389/fnhum.2021.728405. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34751217 Free PMC article.
-
Enhancing Attention Network Spatiotemporal Dynamics for Motor Rehabilitation in Parkinson's Disease.Cyborg Bionic Syst. 2025 Jun 19;6:0293. doi: 10.34133/cbsystems.0293. eCollection 2025. Cyborg Bionic Syst. 2025. PMID: 40538965 Free PMC article.
-
Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus.Int J Neuropsychopharmacol. 2021 May 18;24(5):367-382. doi: 10.1093/ijnp/pyaa097. Int J Neuropsychopharmacol. 2021. PMID: 33315097 Free PMC article. Review.
-
Spatiotemporal EEG microstate analysis in drug-free patients with Parkinson's disease.Neuroimage Clin. 2020;25:102132. doi: 10.1016/j.nicl.2019.102132. Epub 2019 Dec 20. Neuroimage Clin. 2020. PMID: 31884224 Free PMC article.
-
EEG Microstates Change in Response to Increase in Dopaminergic Stimulation in Typical Parkinson's Disease Patients.Front Neurosci. 2018 Oct 15;12:714. doi: 10.3389/fnins.2018.00714. eCollection 2018. Front Neurosci. 2018. PMID: 30374285 Free PMC article.
References
-
- Kesselheim AS, Hwang TJ, Franklin JM. Two decades of new drug development for central nervous system disorders. Nat Rev Drug Discov [Internet]. Nat Publ Group. 2015;14(12):815–6. Available from: http://www.nature.com/doifinder/10.1038/nrd4793. - DOI - PubMed
-
- Kohler I, Hankemeier T, Graaf PH Van Der, Knibbe CAJ, Hasselt JGC Van. European Journal of Pharmaceutical Sciences Integrating clinical metabolomics-based biomarker discovery and clinical pharmacology to enable precision medicine. Eur J Pharm Sci [Internet]. Elsevier; 2017;0–1. 10.1016/j.ejps.2017.05.018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

