Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes
- PMID: 29450670
- PMCID: PMC5814394
- DOI: 10.1186/s40529-018-0222-1
Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes
Abstract
Background: High and low temperatures constitute the most damaging type of abiotic stress and limit the survival, and productivity of plants. The present study aimed to evaluate the role of exogenous applications of acetylsalicylic acid (ASA) in reducing the deleterious effects of cold stress. Phaseolus vulgaris L. seedlings were treated with foliar-sprayed ASA at concentrations of 0-3 mM and then subjected to chilling stress at 4 °C for 2 or 4 days.
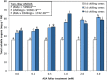
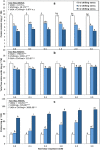
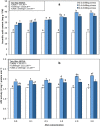
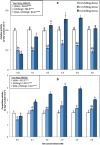
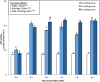
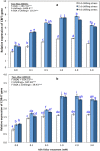
Results: Growth, photosynthesis, biochemical alterations, oxidative damage and antioxidant enzyme activities as well as the expression of cold-responsive genes (CBF3-COR47), were monitored during the experiment. ASA applications substantially improved several growth and photosynthetic parameters, including shoot biomass, dry weight, and photosynthetic pigments, of P. vulgaris seedlings exposed to different durations of chilling stresses. The ASA foliar spray treatments significantly (p < 0.05) rescued the growth and photosynthetic pigments of P. vulgaris seedlings under different chilling stresses. The total soluble sugars markedly increased during 0-4 days of chilling stress following ASA foliar spraying. The exogenous application of ASA significantly (p < 0.05) increased the accumulation of proline in P. vulgaris seedlings under chilling stress. At the gene expression level, ASA significantly (p < 0.05) upregulated the cold-responsive genes CBF3 and COR47.
Conclusions: As a result, we speculate that, the application of exogenous ASA alleviated the adverse effects of chilling stress on all measured parameters, and 1 and 2 mM ASA exhibited the greatest effects.
Keywords: ASA; Acetylsalicylic acid; Antioxidants enzymes; Catalase; Chilling stress; Peroxidase; Superoxide dismutase.
Figures

References
-
- Aebi H (1984) [13] Catalase in vitro. In: Packer L (ed) Methods in enzymology. Academic Press, New York, pp 121–126 - PubMed
-
- Apostolova P, Yordanova R, Popova L. Response of antioxidative defence system to low temperature stress in two wheat cultivars. Gen Appl Plant Physiol. 2008;34:281–294.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

