Endometriosis: clinical features, MR imaging findings and pathologic correlation
- PMID: 29450853
- PMCID: PMC5893487
- DOI: 10.1007/s13244-017-0591-0
Endometriosis: clinical features, MR imaging findings and pathologic correlation
Abstract
Objective: We illustrate the magnetic resonance imaging (MRI) features of endometriosis.
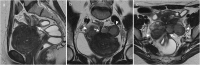
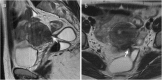
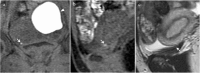
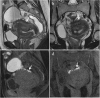
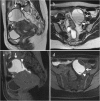
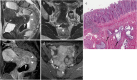
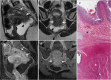
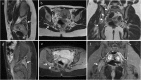
Background: Endometriosis is a chronic gynaecological condition affecting women of reproductive age and may cause pelvic pain and infertility. It is characterized by the growth of functional ectopic endometrial glands and stroma outside the uterus and includes three different manifestations: ovarian endometriomas, peritoneal implants, deep pelvic endometriosis. The primary locations are in the pelvis; extrapelvic endometriosis may rarely occur. Diagnosis requires a combination of clinical history, invasive and non-invasive techniques. The definitive diagnosis is based on laparoscopy with histological confirmation. Diagnostic imaging is necessary for treatment planning. MRI is as a second-line technique after ultrasound. The MRI appearance of endometriotic lesions is variable and depends on the quantity and age of haemorrhage, the amount of endometrial cells, stroma, smooth muscle proliferation and fibrosis. The purpose of surgery is to achieve complete resection of all endometriotic lesions in the same operation.
Conclusion: Owing to the possibility to perform a complete assessment of all pelvic compartments at one time, MRI represents the best imaging technique for preoperative staging of endometriosis, in order to choose the more appropriate surgical approach and to plan a multidisciplinary team work.
Teaching points: • Endometriosis includes ovarian endometriomas, peritoneal implants and deep pelvic endometriosis. • MRI is a second-line imaging technique after US. • Deep pelvic endometriosis is associated with chronic pelvic pain and infertility. • Endometriosis is characterized by considerable diagnostic delay. • MRI is the best imaging technique for preoperative staging of endometriosis.
Keywords: Deep infiltrating endometriosis; Endometrioma; Endometriosis; Magnetic resonance imaging; Pelvic pain; Pelvis.
Figures
















References
-
- Adamson GD, Kennedy SH, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the world endometriosis research foundation. J Endometriosis. 2010;2:3–6.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

