m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration
- PMID: 29451229
- PMCID: PMC5835776
- DOI: 10.1038/cr.2018.17
m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration
Abstract
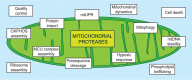
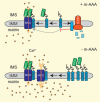
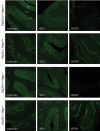
The function of mitochondria depends on ubiquitously expressed and evolutionary conserved m-AAA proteases in the inner membrane. These ATP-dependent peptidases form hexameric complexes built up of homologous subunits. AFG3L2 subunits assemble either into homo-oligomeric isoenzymes or with SPG7 (paraplegin) subunits into hetero-oligomeric proteolytic complexes. Mutations in AFG3L2 are associated with dominant spinocerebellar ataxia (SCA28) characterized by the loss of Purkinje cells, whereas mutations in SPG7 cause a recessive form of hereditary spastic paraplegia (HSP7) with motor neurons of the cortico-spinal tract being predominantly affected. Pleiotropic functions have been assigned to m-AAA proteases, which act as quality control and regulatory enzymes in mitochondria. Loss of m-AAA proteases affects mitochondrial protein synthesis and respiration and leads to mitochondrial fragmentation and deficiencies in the axonal transport of mitochondria. Moreover m-AAA proteases regulate the assembly of the mitochondrial calcium uniporter (MCU) complex. Impaired degradation of the MCU subunit EMRE in AFG3L2-deficient mitochondria results in the formation of deregulated MCU complexes, increased mitochondrial calcium uptake and increased vulnerability of neurons for calcium-induced cell death. A reduction of calcium influx into the cytosol of Purkinje cells rescues ataxia in an AFG3L2-deficient mouse model. In this review, we discuss the relationship between the m-AAA protease and mitochondrial calcium homeostasis and its relevance for neurodegeneration and describe a novel mouse model lacking MCU specifically in Purkinje cells. Our results pledge for a novel view on m-AAA proteases that integrates their pleiotropic functions in mitochondria to explain the pathogenesis of associated neurodegenerative disorders.
Figures
References
-
- Quiros PM, Langer T, Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol 2015; 16:345–359. - PubMed
-
- Gerdes F, Tatsuta T, Langer T. Mitochondrial AAA proteases - towards a molecular understanding of membrane-bound proteolytic machines. Biochim Biophys Acta 2012; 1823:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

