MicroRNA-365 modulates astrocyte conversion into neuron in adult rat brain after stroke by targeting Pax6
- PMID: 29451327
- PMCID: PMC6001668
- DOI: 10.1002/glia.23308
MicroRNA-365 modulates astrocyte conversion into neuron in adult rat brain after stroke by targeting Pax6
Abstract
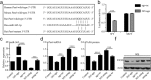
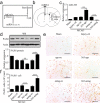
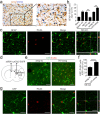
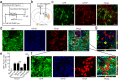
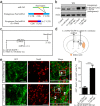
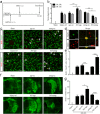
Reactive astrocytes induced by ischemia can transdifferentiate into mature neurons. This neurogenic potential of astrocytes may have therapeutic value for brain injury. Epigenetic modifications are widely known to involve in developmental and adult neurogenesis. PAX6, a neurogenic fate determinant, contributes to the astrocyte-to-neuron conversion. However, it is unclear whether microRNAs (miRs) modulate PAX6-mediated astrocyte-to-neuron conversion. In the present study we used bioinformatic approaches to predict miRs potentially targeting Pax6, and transient middle cerebral artery occlusion (MCAO) to model cerebral ischemic injury in adult rats. These rats were given striatal injection of glial fibrillary acidic protein targeted enhanced green fluorescence protein lentiviral vectors (Lv-GFAP-EGFP) to permit cell fate mapping for tracing astrocytes-derived neurons. We verified that miR-365 directly targets to the 3'-UTR of Pax6 by luciferase assay. We found that miR-365 expression was significantly increased in the ischemic brain. Intraventricular injection of miR-365 antagomir effectively increased astrocytic PAX6 expression and the number of new mature neurons derived from astrocytes in the ischemic striatum, and reduced neurological deficits as well as cerebral infarct volume. Conversely, miR-365 agomir reduced PAX6 expression and neurogenesis, and worsened brain injury. Moreover, exogenous overexpression of PAX6 enhanced the astrocyte-to-neuron conversion and abolished the effects of miR-365. Our results demonstrate that increase of miR-365 in the ischemic brain inhibits astrocyte-to-neuron conversion by targeting Pax6, whereas knockdown of miR-365 enhances PAX6-mediated neurogenesis from astrocytes and attenuates neuronal injury in the brain after ischemic stroke. Our findings provide a foundation for developing novel therapeutic strategies for brain injury.
Keywords: astrocyte; brain repair; glia; ischemic injury; microRNA; neurogenesis; neuron.
© 2018 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures


References
-
- Allen, N. J. , & Barres, B. A. (2009). NEUROSCIENCE Glia – More than just brain glue. Nature, 457(7230), 675–677. https://doi.org/10.1038/457675a - DOI - PubMed
-
- Alvarez, J. I. , Katayama, T. , & Prat, A. (2013). Glial influence on the blood brain barrier. Glia, 61(12), 1939–1958. https://doi.org/10.1002/glia.22575 - DOI - PMC - PubMed
-
- Arvidsson, A. , Collin, T. , Kirik, D. , Kokaia, Z. , & Lindvall, O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Medicine, 8(9), 963–970. https://doi.org/10.1038/nm747 - DOI - PubMed
-
- Bartel, D. P. (2007). MicroRNAs: Genomics, biogenesis, mechanism, and function (Reprinted from Cell, vol 116, pg 281–297, 2004). Cell, 131(4), 11–29. https://doi.org/10.1016/S0092-8674(04)00045-5 - DOI - PubMed
-
- Bhalala, O. G. , Srikanth, M. , & Kessler, J. A. (2013). The emerging roles of microRNAs in CNS injuries. Nature Reviews Neurology, 9(6), 328–339. https://doi.org/10.1038/nrneurol.2013.67 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

