Intracellular pharmacokinetics of gemcitabine, its deaminated metabolite 2',2'-difluorodeoxyuridine and their nucleotides
- PMID: 29451684
- PMCID: PMC5980516
- DOI: 10.1111/bcp.13557
Intracellular pharmacokinetics of gemcitabine, its deaminated metabolite 2',2'-difluorodeoxyuridine and their nucleotides
Abstract
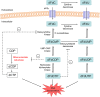
Aims: Gemcitabine (2',2'-difluoro-2'-deoxycytidine; dFdC) is a prodrug that has to be phosphorylated within the tumour cell to become active. Intracellularly formed gemcitabine diphosphate (dFdCDP) and triphosphate (dFdCTP) are considered responsible for the antineoplastic effects of gemcitabine. However, a major part of gemcitabine is converted into 2',2'-difluoro-2'-deoxyuridine (dFdU) by deamination. In the cell, dFdU can also be phosphorylated to its monophosphate (dFdUMP), diphosphate (dFdUDP) and triphosphate (dFdUTP). In vitro data suggest that these dFdU nucleotides might also contribute to the antitumour effects, although little is known about their intracellular pharmacokinetics (PK). Therefore, the objective of the present study was to gain insight into the intracellular PK of all dFdC and dFdU nucleotides formed during gemcitabine treatment.
Methods: Peripheral blood mononuclear cell (PBMC) samples were collected from 38 patients receiving gemcitabine, at multiple time points after infusion. Gemcitabine, dFdU and their nucleotides were quantified in PBMCs. In addition, gemcitabine and dFdU plasma concentrations were monitored. The individual PK parameters in plasma and in PBMCs were determined.
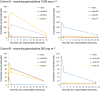
Results: Both in plasma and in PBMCs, dFdU was present in higher concentrations than gemcitabine [mean intracellular area under the concentration-time curve from time zero to 24 h (AUC0-24 h ) 1650 vs. 95 μM*h]. However, the dFdUMP, dFdUDP and dFdUTP concentrations in PBMCs were much lower than the dFdCDP and dFdCTP concentrations. The mean AUC0-24 h for dFdUTP was 312 μM*h vs. 2640 μM*h for dFdCTP.
Conclusions: The study provides the first complete picture of all nucleotides that are formed intracellularly during gemcitabine treatment. Low intracellular dFdU nucleotide concentrations were found, which calls into question the relevance of these nucleotides for the cytotoxic effects of gemcitabine.
Keywords: 2′,2′-difluorodeoxycytidine triphosphate (dFdCTP); 2′,2′-difluorodeoxyuridine triphosphate (dFdUTP); gemcitabine; nucleotides; peripheral blood mononuclear cells (PBMCs); pharmacokinetics.
© 2018 The British Pharmacological Society.
Figures

References
-
- Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, et al Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res 1998; 58: 4349–4357. - PubMed
-
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al Recent molecular advances in studies of the concentrative Na+‐dependent nucleoside transporter (CNT) family: identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides. Mol Membr Biol 2001; 18: 65–72. - PubMed
-
- Wong A, Soo RA, Yong W‐P, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev 2009; 41: 77–88. - PubMed
-
- Gandhi V, Legha J, Chen F, Hertel LW, Plunkett W. Excision of 2′,2′‐difluorodeoxycytidine (gemcitabine) monophosphate residues from DNA. Cancer Res 1996; 56: 4453–4459. - PubMed
-
- Huang P, Chubb S, Hertel L, Grindey G, Plunkett W. Action of 2′,2′‐difluorodeoxycytidine on DNA synthesis. Cancer Res 1991; 51: 6110–6117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

