Short-Term Pulmonary Toxicity Assessment of Pre- and Post-incinerated Organomodified Nanoclay in Mice
- PMID: 29451776
- PMCID: PMC6357971
- DOI: 10.1021/acsnano.7b07281
Short-Term Pulmonary Toxicity Assessment of Pre- and Post-incinerated Organomodified Nanoclay in Mice
Abstract
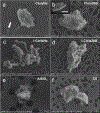
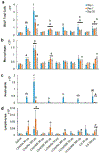
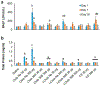
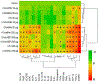
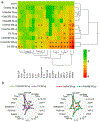
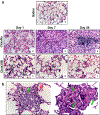
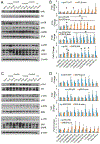
Organomodified nanoclays (ONCs) are increasingly used as filler materials to improve nanocomposite strength, wettability, flammability, and durability. However, pulmonary risks associated with exposure along their chemical lifecycle are unknown. This study's objective was to compare pre- and post-incinerated forms of uncoated and organomodified nanoclays for potential pulmonary inflammation, toxicity, and systemic blood response. Mice were exposed via aspiration to low (30 μg) and high (300 μg) doses of preincinerated uncoated montmorillonite nanoclay (CloisNa), ONC (Clois30B), their respective incinerated forms (I-CloisNa and I-Clois30B), and crystalline silica (CS). Lung and blood tissues were collected at days 1, 7, and 28 to compare toxicity and inflammation indices. Well-dispersed CloisNa caused a robust inflammatory response characterized by neutrophils, macrophages, and particle-laden granulomas. Alternatively, Clois30B, I-Clois30B, and CS high-dose exposures elicited a low grade, persistent inflammatory response. High-dose Clois30B exposure exhibited moderate increases in lung damage markers and a delayed macrophage recruitment cytokine signature peaking at day 7 followed by a fibrotic tissue signature at day 28, similar to CloisNa. I-CloisNa exhibited acute, transient inflammation with quick recovery. Conversely, high-dose I-Clois30B caused a weak initial inflammatory signal but showed comparable pro-inflammatory signaling to CS at day 28. The data demonstrate that ONC pulmonary toxicity and inflammatory potential relies on coating presence and incineration status in that coated and incinerated nanoclay exhibited less inflammation and granuloma formation than pristine montmorillonite. High doses of both pre- and post-incinerated ONC, with different surface morphologies, may harbor potential pulmonary health hazards over long-term occupational exposures.
Keywords: inflammation; life cycle; nanoparticles; pulmonary; toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Patel HA; Somani RS; Bajaj HC; Jasra RV Nanoclays for Polymer Nanocomposites, Paints, Inks, Greases and Cosmetics Formulations, Drug Delivery Vehicle and Waste Water Treatment. Bull. Mater. Sci 2006, 29, 133–145.
-
- Anandhan S; Bandyopadhyay S Polymer Nanocomposites: From Synthesis to Applications. In Nanocomposites and polymers with analytical methods; Cuppoletti, J., Ed.; InTechOpen, 2011; pp 1–27.
-
- Camargo PHC; Satyanarayana KG; Wypych F Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res 2009, 12, 1–39.
-
- Mackevica A; Foss Hansen S Release of Nanomaterials from Solid Nanocomposites and Consumer Exposure Assessment- A Forward-Looking Review. Nanotoxicology 2016, 10, 641–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

