Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline
- PMID: 29451817
- PMCID: PMC6032083
- DOI: 10.1152/ajpheart.00637.2017
Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline
Abstract
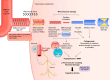
Aging is a modern concept: human life expectancy has more than doubled in less than 150 yr in Western countries. Longer life span, however, reveals age-related diseases, including cerebrovascular diseases. The vascular system is a prime target of aging: the "wear and tear" of large elastic arteries exposed to a lifelong pulsatile pressure causes arterial stiffening by fragmentation of elastin fibers and replacement by stiffer collagen. This arterial stiffening increases in return the amplitude of the pulse pressure (PP), its wave penetrating deeper into the microcirculation of low-resistance, high-flow organs such as the brain. Several studies have associated peripheral arterial stiffness responsible for the sustained increase in PP, with brain microvascular diseases such as cerebral small vessel disease, cortical gray matter thinning, white matter atrophy, and cognitive dysfunction in older individuals and prematurely in hypertensive and diabetic patients. The rarefaction of white matter is also associated with middle cerebral artery pulsatility that is strongly dependent on PP and artery stiffness. PP and brain damage are likely associated, but the sequence of mechanistic events has not been established. Elevated PP promotes endothelial dysfunction that may slowly develop in parallel with the accumulation of proinflammatory senescent cells and oxidative stress, generating cerebrovascular damage and remodeling, as well as brain structural changes. Here, we review data suggesting that age-related increased peripheral artery stiffness may promote the penetration of a high PP to cerebral microvessels, likely causing functional, structural, metabolic, and hemodynamic alterations that could ultimately promote neuronal dysfunction and cognitive decline.
Keywords: cerebral arteries; endothelium; large elastic arteries; pulsatile pressure and flow.
Figures

References
-
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jónsdottir KY, Møller A, Ashkanian M, Vafaee MS, Iversen P, Johannsen P, Gjedde A. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 32: 1177–1187, 2012. doi: 10.1038/jcbfm.2012.18. - DOI - PMC - PubMed
-
- Akima M, Nonaka H, Kagesawa M, Tanaka K. A study on the microvasculature of the cerebral cortex. Fundamental architecture and its senile change in the frontal cortex. Lab Invest 55: 482–489, 1986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

