3D bioprinted functional and contractile cardiac tissue constructs
- PMID: 29452273
- PMCID: PMC6022829
- DOI: 10.1016/j.actbio.2018.02.007
3D bioprinted functional and contractile cardiac tissue constructs
Abstract
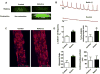
Bioengineering of a functional cardiac tissue composed of primary cardiomyocytes has great potential for myocardial regeneration and in vitro tissue modeling. However, its applications remain limited because the cardiac tissue is a highly organized structure with unique physiologic, biomechanical, and electrical properties. In this study, we undertook a proof-of-concept study to develop a contractile cardiac tissue with cellular organization, uniformity, and scalability by using three-dimensional (3D) bioprinting strategy. Primary cardiomyocytes were isolated from infant rat hearts and suspended in a fibrin-based bioink to determine the priting capability for cardiac tissue engineering. This cell-laden hydrogel was sequentially printed with a sacrificial hydrogel and a supporting polymeric frame through a 300-µm nozzle by pressured air. Bioprinted cardiac tissue constructs had a spontaneous synchronous contraction in culture, implying in vitro cardiac tissue development and maturation. Progressive cardiac tissue development was confirmed by immunostaining for α-actinin and connexin 43, indicating that cardiac tissues were formed with uniformly aligned, dense, and electromechanically coupled cardiac cells. These constructs exhibited physiologic responses to known cardiac drugs regarding beating frequency and contraction forces. In addition, Notch signaling blockade significantly accelerated development and maturation of bioprinted cardiac tissues. Our results demonstrated the feasibility of bioprinting functional cardiac tissues that could be used for tissue engineering applications and pharmaceutical purposes.
Statement of significance: Cardiovascular disease remains a leading cause of death in the United States and a major health-care burden. Myocardial infarction (MI) is a main cause of death in cardiovascular diseases. MI occurs as a consequence of sudden blocking of blood vessels supplying the heart. When occlusions in the coronary arteries occur, an immediate decrease in nutrient and oxygen supply to the cardiac muscle, resulting in permanent cardiac cell death. Eventually, scar tissue formed in the damaged cardiac muscle that cannot conduct electrical or mechanical stimuli thus leading to a reduction in the pumping efficiency of the heart. The therapeutic options available for end-stage heart failure is to undergo heart transplantation or the use of mechanical ventricular assist devices (VADs). However, many patients die while being on a waiting list, due to the organ shortage and limitation of VADs, such as surgical complications, infection, thrombogenesis, and failure of the electrical motor and hemolysis. Ultimately, 3D bioprinting strategy aims to create clinically applicable tissue constructs that can be immediately implanted in the body. To date, the focus on replicating complex and heterogeneous tissue constructs continues to increase as 3D bioprinting technologies advance. In this study, we demonstrated the feasibility of 3D bioprinting strategy to bioengineer the functional cardiac tissue that possesses a highly organized structure with unique physiological and biomechanical properties similar to native cardiac tissue. This bioprinting strategy has great potential to precisely generate functional cardiac tissues for use in pharmaceutical and regenerative medicine applications.
Keywords: Bioprinting; Body-on-a-chip; Cardiac tissue; Contractility; Heart failure; In vitro tissue model; Tissue engineering.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, C. American Heart Association Statistics, S. Stroke Statistics Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. - PMC - PubMed
-
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. - PubMed
-
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–458. - PubMed
-
- Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Bellabas L, Brinon B, Vanneaux V, Pradeau P, Peyrard S, Larghero J, Pouly J, Binder P, Garcia S, Shimizu T, Sawa Y, Okano T, Bruneval P, Desnos M, Hagege AA, Casteilla L, Puceat M, Menasche P. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122(11 Suppl):S118–S123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

