Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels
- PMID: 29452287
- PMCID: PMC5972055
- DOI: 10.1016/j.spinee.2018.02.007
Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels
Abstract
Background context: Advances in the development of biomaterials and stem cell therapy provide a promising approach to regenerating degenerated discs. The normal nucleus pulposus (NP) cells exhibit similar phenotype to chondrocytes. Because dental pulp stem cells (DPSCs) can be differentiated into chondrogenic cells, the DPSCs and DPSCs-derived chondrogenic cells encapsulated in type I and type II collagen hydrogels can potentially be transplanted into degenerated NP to repair damaged tissue. The motility of transplanted cells is critical because the cells need to migrate away from the hydrogels containing the cells of high density and disperse through the NP tissue after implantation.
Purpose: The purpose of this study was to determine the motility of DPSC and DPSC-derived chondrogenic cells in type I and type II collagen hydrogels.
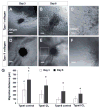
Study design/setting: The time lapse imaging that recorded cell migration was analyzed to quantify the cell migration velocity and distance.
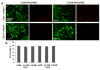
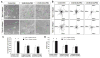
Methods: The cell viability of DPSCs in native or poly(ethylene glycol) ether tetrasuccinimidyl glutarate (4S-StarPEG)-crosslinked type I and type II collagen hydrogels was determined using LIVE/DEAD cell viability assay and AlamarBlue assay. DPSCs were differentiated into chondrogenic cells. The migration of DPSCs and DPSC-derived chondrogenic cells in these hydrogels was recorded using a time lapse imaging system. This study was funded by the Regional Institute on Aging and Wichita Medical Research and Education Foundation, and the authors declare no competing interest.
Result: DPSCs showed high cell viability in non-crosslinked and crosslinked collagen hydrogels. DPSCs migrated in collagen hydrogels, and the cell migration speed was not significantly different in either type I collagen or type II collagen hydrogels. The migration speed of DPSC-derived chondrogenic cells was higher in type I collagen hydrogel than in type II collagen hydrogel. Crosslinking of type I collagen with 4S-StarPEG significantly reduced the cell migration speed of DPSC-derived chondrogenic cells.
Conclusions: After implantation of collagen hydrogels encapsulating DPSCs or DPSC-derived chondrogenic cells, the cells can potentially migrate from the hydrogels and migrate into the NP tissue. This study also explored the differential cell motility of DPSCs and DPSC-derived chondrogenic cells in these collagen hydrogels.
Keywords: Chondrogenic cell; Collagen; Dental pulp stem cells; Migration; Nucleus pulposus; Regeneration.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures




References
-
- Gan Y, Li P, Wang L, Mo X, Song L, Xu Y, Zhao C, Ouyang B, Tu B, Luo L, Zhu L, Dong S, Li F, Zhou Q. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials. 2017 Aug;136:12–28. doi: 10.1016/j.biomaterials.2017.05.017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

