Repetitive TLR3 activation in the lung induces skeletal muscle adaptations and cachexia
- PMID: 29452288
- PMCID: PMC5911410
- DOI: 10.1016/j.exger.2018.02.002
Repetitive TLR3 activation in the lung induces skeletal muscle adaptations and cachexia
Abstract
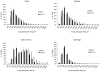
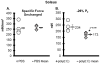
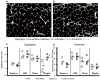
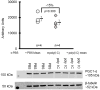
Due to immunosenescence, older adults are particularly susceptible to lung-based viral infections, with increased severity of symptoms in those with underlying chronic lung disease. Repeated respiratory viral infections produce lung maladaptations, accelerating pulmonary dysfunction. Toll like 3 receptor (TLR3) is a membrane protein that senses exogenous double-stranded RNA to activate the innate immune response to a viral infection. Polyinosinic-polycytidylic acid [poly(I:C)] mimics double stranded RNA and has been shown to activate TLR3. Utilizing an established mouse viral exacerbation model produced by repetitive intranasal poly(I:C) administration, we sought to determine whether repetitive poly(I:C) treatment induced negative muscle adaptations (i.e. atrophy, weakness, and loss of function). We determined skeletal muscle morphological properties (e.g. fiber-type, fiber cross-sectional area, muscle wet mass, etc.) from a treated group ((poly(I:C), n = 9) and a sham-treated control group (PBS, n = 9); age approximately 5 months. In a subset (n = 4 for both groups), we determined in vivo physical function (using grip test for strength, rotarod for overall motor function, and treadmill for endurance) and muscle contractile properties with in vitro physiology (in the EDL, soleus and diaphragm). Our findings demonstrate that poly(I:C)-treated mice exhibit both muscle morphological and functional deficits. Changes of note when comparing poly(I:C)-treated mice to PBS-treated controls include reductions in fiber cross-sectional area (-27% gastrocnemius, -25% soleus, -16% diaphragm), contractile dysfunction (soleus peak tetanic force, -26%), muscle mass (gastrocnemius -19%, soleus -23%), physical function (grip test -34%), body mass (-20%), and altered oxidative capacity (140% increase in succinate dehydrogenase activity in the diaphragm, but 66% lower in the gastrocnemius). Our data is supportive of a new model of cachexia/sarcopenia that has potential for future research into the mechanisms underlying muscle wasting.
Keywords: Ageing; COPD cachexia; Mouse models; Physical function; Skeletal muscle; TLR3.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest:
The authors report no conflicts of interest whether financial or otherwise.
Figures

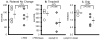




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

