Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts
- PMID: 29452461
- PMCID: PMC5985151
- DOI: 10.1016/j.jtcvs.2017.08.127
Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts
Abstract
Objective: Although the mammalian heart's ability to fully regenerate is debated, its potential to extensively repair itself is gaining support. We hypothesized that heart regeneration relies on rapid angiogenesis to support myocardial regrowth and sought to characterize the timeline for angiogenesis and cell proliferation in regeneration.
Methods: One-day-old CD-1 mice (P1, N = 60) underwent apical resection or sham surgery. Hearts were explanted at serial time points from 0 to 30 days postresection and analyzed with immunohistochemistry to visualize vessel ingrowth and cardiomyocyte migration into the resected region. Proliferating cells were labeled with 5-ethynyl-2'-deoxyuridine injections 12 hours before explant. 5-Ethynyl-2'-deoxyuridine-positive cells were counted in both the apex and remote areas of the heart. Masson's trichrome was used to assess fibrosis.
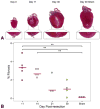
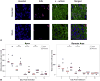
Results: By 30 days postresection, hearts regenerated with minimal fibrosis. Compared with sham surgery, apical resection stimulated a significant increase in proliferation of preexisting cardiomyocytes between 3 and 11 days after injury. Capillary migration into the apical thrombus was detected as early as 2 days postresection, with development of mature arteries by 5 days postresection. New vessels became perfused by 5 days postresection as evidenced by lectin injection. Vessel density and diameter significantly increased within the resected area over 21 days, and vessel ingrowth always preceded cardiomyocyte migration, with coalignment of most migrating cardiomyocytes with ingrowing vessels.
Conclusions: Endothelial cells migrate into the apical thrombus early after resection, develop into functional arteries, and precede cardiomyocyte ingrowth during mammalian heart regeneration. This endogenous neonatal response emphasizes the importance of expeditious angiogenesis required for neomyogenesis.
Keywords: angiogenesis; arteriogenesis; cardiac regeneration; cardiomyocyte migration; vascular biology.
Copyright © 2017 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Heart regeneration: The endothelial cell comes first.J Thorac Cardiovasc Surg. 2018 Mar;155(3):1128-1129. doi: 10.1016/j.jtcvs.2017.09.106. Epub 2017 Oct 3. J Thorac Cardiovasc Surg. 2018. PMID: 29452462 Free PMC article. No abstract available.
-
Successful rebuilding after disaster, even in the heart, starts with infrastructure.J Thorac Dis. 2018 Nov;10(Suppl 33):S4165-S4167. doi: 10.21037/jtd.2018.10.95. J Thorac Dis. 2018. PMID: 30631583 Free PMC article. No abstract available.
References
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. - PubMed
-
- Witman N, Murtuza B, Davis B, Arner A, Morrison JI. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol. 2011;354:67–76. - PubMed
-
- Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–59. - PubMed
-
- Tam SKC, Gu W, Mahdavi V, Nadal-Ginard B. Cardiac myocyte terminal differentiation. Ann N Y Acad Sci. 1995;752:72–9. - PubMed
-
- Li FQ, Wang XJ, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Car-diol. 1996;28:1737–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

